Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
GASTROENTEROLOGY / RESEARCH PAPER
Early and simple laboratory markers of poor outcome in children with IBD.
1
Department of Pediatrics, Gastroenterology, Hepatology, Nutrition, Allergology and Pulmonology, Medical University of Bialystok, Bialystok, Poland
Submission date: 2025-03-26
Final revision date: 2025-04-27
Acceptance date: 2025-05-11
Online publication date: 2025-06-23
Corresponding author
Katarzyna Zdanowicz
Department of Pediatrics, Gastroenterology, Hepatology, Nutrition, Allergology and Pulmonology, Medical University of Bialystok, Bialystok, Poland, Poland
Department of Pediatrics, Gastroenterology, Hepatology, Nutrition, Allergology and Pulmonology, Medical University of Bialystok, Bialystok, Poland, Poland
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Personalized medicine in inflammatory bowel disease (IBD) aims to achieve maximum effectiveness through rapid induction and maintenance of remission. To achieve this goal, reliable predictors of disease course are needed. The aim of this study was to identify early markers of IBD's poor course understood as the need for anti-tumor necrosis factor (anti-TNFα) treatment.
Material and methods:
We analyzed the clinical, laboratory, radiological and endoscopic data of children with IBD. These parameters were assessed at the time of diagnosis (T0) and 8-12 weeks (T1) after the start of induction therapy. The results of patients who did not require anti-TNFα treatment were compared to children who needed such treatment during the 2-year observation time.
Results:
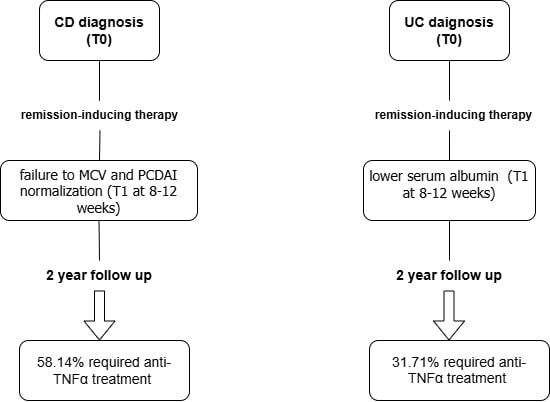
58.14% of patients with Crohn's disease (CD) and 31.71% of patients with ulcerative colitis (UC) required biological therapy. Patients with CD and UC receiving biological therapy, compared with those without, differed in selected clinical and laboratory parameters both at T0 and T1. In multivariate analysis, the risk of anti-TNFα therapy in patients with CD was associated with the lack of normalization of MCV and PCDAI. In patients with UC higher albumin levels reduced this risk.
Conclusions:
In children with IBD, disease activity and concentrations of selected biochemical parameters assessed within 3 months after diagnosis may be helpful in predicting a poor outcome of CD and UC.
Personalized medicine in inflammatory bowel disease (IBD) aims to achieve maximum effectiveness through rapid induction and maintenance of remission. To achieve this goal, reliable predictors of disease course are needed. The aim of this study was to identify early markers of IBD's poor course understood as the need for anti-tumor necrosis factor (anti-TNFα) treatment.
Material and methods:
We analyzed the clinical, laboratory, radiological and endoscopic data of children with IBD. These parameters were assessed at the time of diagnosis (T0) and 8-12 weeks (T1) after the start of induction therapy. The results of patients who did not require anti-TNFα treatment were compared to children who needed such treatment during the 2-year observation time.
Results:
58.14% of patients with Crohn's disease (CD) and 31.71% of patients with ulcerative colitis (UC) required biological therapy. Patients with CD and UC receiving biological therapy, compared with those without, differed in selected clinical and laboratory parameters both at T0 and T1. In multivariate analysis, the risk of anti-TNFα therapy in patients with CD was associated with the lack of normalization of MCV and PCDAI. In patients with UC higher albumin levels reduced this risk.
Conclusions:
In children with IBD, disease activity and concentrations of selected biochemical parameters assessed within 3 months after diagnosis may be helpful in predicting a poor outcome of CD and UC.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.