Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
Effect of immune cells and plasma metabolites on osteomyelitis: a two-sample Mendelian randomization and mediation analysis
1
Department of Orthopedics and Traumatology, Shuguang-Anhui Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Western Area of the First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, China
Submission date: 2024-11-11
Final revision date: 2025-03-11
Acceptance date: 2025-04-06
Online publication date: 2025-07-03
Corresponding author
Guang Yang
Department of Orthopedics and Traumatology Shuguang-Anhui Hospital Affiliated to Shanghai University of Traditional Chinese Medicine Western Area of the First Affiliated Hospital of Anhui University of Chinese Medicine Hefei, Anhui, China
Department of Orthopedics and Traumatology Shuguang-Anhui Hospital Affiliated to Shanghai University of Traditional Chinese Medicine Western Area of the First Affiliated Hospital of Anhui University of Chinese Medicine Hefei, Anhui, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Osteomyelitis (OM) is an infectious bone disease characterized by complex immune and metabolic features. Previous studies have found that immune cells play an important role in the development and progression of OM. However, the causal relationship between immune cells, plasma metabolites, and OM remains undetermined.
Material and methods:
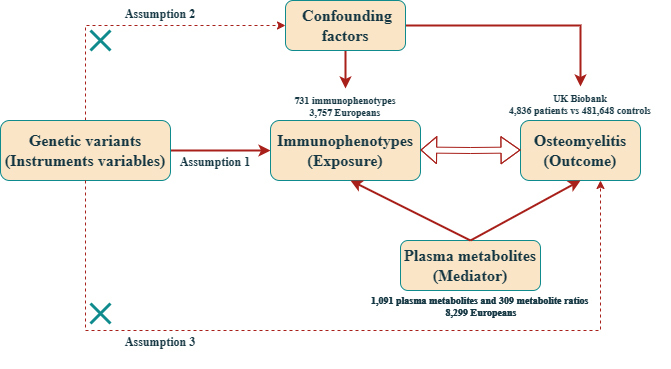
Instrumental variables (IVs) for 1400 plasma metabolite features (N = 7,824) and 731 immunophenotypes (N = 3,757) were sourced from genome-wide association studies (GWAS). The IVs for OM were derived from a comprehensive GWAS meta-analysis dataset of European ancestry. The relationship between exposure and outcome was assessed using two-sample Mendelian randomization (MR) analysis. The robustness of the results was evaluated through heterogeneity tests, sensitivity analyses, and pleiotropy analyses. Additionally, mediation analysis was employed to identify pathways through which immune characteristics and metabolites mediate OM.
Results:
MR analysis revealed a genetic causal relationship between three immunophenotypes and nine plasma metabolites with OM. Reverse MR was used to identify the directionality of the causal relationship between CD27 on switched memory B cells, CD127 on CD8+ T cells, and OM. Lastly, mediation analysis confirmed that three plasma metabolites have a significant mediating effect on the association between two immune phenotypes and OM.
Conclusions:
Through MR analysis, this study demonstrated that plasma metabolites can mediate the causal effects of immune phenotypes on OM, providing new insights into the development mechanisms of OM and potential biomarkers, which hold promising value for the clinical diagnosis and treatment of OM.
Osteomyelitis (OM) is an infectious bone disease characterized by complex immune and metabolic features. Previous studies have found that immune cells play an important role in the development and progression of OM. However, the causal relationship between immune cells, plasma metabolites, and OM remains undetermined.
Material and methods:
Instrumental variables (IVs) for 1400 plasma metabolite features (N = 7,824) and 731 immunophenotypes (N = 3,757) were sourced from genome-wide association studies (GWAS). The IVs for OM were derived from a comprehensive GWAS meta-analysis dataset of European ancestry. The relationship between exposure and outcome was assessed using two-sample Mendelian randomization (MR) analysis. The robustness of the results was evaluated through heterogeneity tests, sensitivity analyses, and pleiotropy analyses. Additionally, mediation analysis was employed to identify pathways through which immune characteristics and metabolites mediate OM.
Results:
MR analysis revealed a genetic causal relationship between three immunophenotypes and nine plasma metabolites with OM. Reverse MR was used to identify the directionality of the causal relationship between CD27 on switched memory B cells, CD127 on CD8+ T cells, and OM. Lastly, mediation analysis confirmed that three plasma metabolites have a significant mediating effect on the association between two immune phenotypes and OM.
Conclusions:
Through MR analysis, this study demonstrated that plasma metabolites can mediate the causal effects of immune phenotypes on OM, providing new insights into the development mechanisms of OM and potential biomarkers, which hold promising value for the clinical diagnosis and treatment of OM.
REFERENCES (40)
1.
Momodu II, Savaliya V. Osteomyelitis. StatPearls. Treasure Island (FL): StatPearls Publishing, StatPearls Publishing LLC.; 2022.
4.
Wang X, Zhang M, Zhu T, Wei Q, Liu G, Ding J. Flourishing antibacterial strategies for osteomyelitis therapy. Adv Sci 2023; 10: e2206154.
5.
Deng Z, Cai L, Jin W, Ping A, Wei R. One-stage reconstruction with open bone grafting and vacuum-assisted closure for infected tibial non-union. Arch Med Sci 2014; 10: 764-72.
7.
Shen CJ, Wu MS, Lin KH, et al. The use of procalcitonin in the diagnosis of bone and joint infection: a systemic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2013; 32: 807-14.
8.
Masters EA, Ricciardi BF, Bentley KLM, Moriarty TF, Schwarz EM, Muthukrishnan G. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol 2022; 20: 385-400.
9.
Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 2012; 34: 237-59.
10.
Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 2017; 15: 435-47.
11.
Bröker BM, Mrochen D, Péton V. The T cell response to Staphylococcus aureus. Pathogens 2016; 5: 31.
12.
Lee B, Olaniyi R, Kwiecinski JM, Wardenburg JB. Staphylococcus aureus toxin suppresses antigen-specific T cell responses. J Clin Invest 2020; 130: 1122-7.
13.
Zhang B, Su Y, Zhou J, Zheng Y, Zhu D. Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses. Adv Sci (Weinh) 2021; 8: e2100446.
14.
Mysliwiec H, Harasim-Symbor E, Baran A, et al. Abnormal serum fatty acid profile in psoriatic arthritis. Arch Med Sci 2019; 15: 1407-14.
15.
Schirmer M, Stražar M, Avila-Pacheco J, et al. Linking microbial genes to plasma and stool metabolites uncovers host-microbial interactions underlying ulcerative colitis disease course. Cell Host Microbe 2024; 32: 209-26.e7.
16.
Isogai N, Shiono Y, Kuramoto T, et al. Potential osteomyelitis biomarkers identified by plasma metabolome analysis in mice. Sci Rep 2020; 10: 839.
17.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008; 27: 1133-63.
18.
Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003; 32: 1-22.
19.
Orrù V, Steri M, Sidore C, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet 2020; 52: 1036-45.
20.
Chen Y, Lu T, Pettersson-Kymmer U, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet 2023; 55: 44-53.
21.
Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779.
22.
Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018; 7: e34408.
23.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016; 40: 304-14.
24.
Carter AR, Sanderson E, Hammerton G, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 2021; 36: 465-78.
25.
Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM. Mechanisms of immune evasion and bone tissue colonization that make staphylococcus aureus the primary pathogen in osteomyelitis. Curr Osteoporos Rep 2019; 17: 395-404.
26.
Kumar G, Roger PM, Ticchioni M, et al. T cells from chronic bone infection show reduced proliferation and a high proportion of CD28⁻ CD4 T cells. Clin Exp Immunol 2014; 176: 49-57.
27.
Sokhi UK, Xia Y, Sosa B, et al. Immune response to persistent Staphyloccocus aureus periprosthetic joint infection in a mouse tibial implant model. J Bone Miner Res 2022; 37: 577-94.
28.
Mais DD, Hackman S, Ross J. Histopathologic findings in culture-positive secondary osteomyelitis. Ann Diagn Pathol 2021; 50: 151661.
29.
Wagner C, Heck D, Lautenschläger K, et al. T lymphocytes in implant-associated posttraumatic osteomyelitis: identification of cytotoxic T effector cells at the site of infection. Shock 2006; 25: 241-6.
30.
Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol 1999; 30: 59-65.
31.
Meednu N, Zhang H, Owen T, et al. Production of RANKL by memory B cells: a link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 805-16.
32.
Wagner JM, Jaurich H, Wallner C, et al. Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity, and increased osteoclast activity. J Orthop Res 2017; 35: 2425-34.
33.
Wagner JM, Reinkemeier F, Wallner C, et al. Adipose-derived stromal cells are capable of restoring bone regeneration after post-traumatic osteomyelitis and modulate B-cell response. Stem Cells Transl Med 2019; 8: 1084-91.
34.
Bauermeister A, Mannochio-Russo H, Costa-Lotufo LV, Jarmusch AK, Dorrestein PC. Mass spectrometry-based metabolomics in microbiome investigations. Nat Rev Microbiol 2022; 20: 143-60.
35.
Baidoo EEK, Teixeira Benites V. Mass spectrometry-based microbial metabolomics: techniques, analysis, and applications. Methods Mol Biol 2019; 1859: 11-69.
36.
Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006; 83(6 Suppl): 1505s-19s.
37.
Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 2014; 141: 272-82.
38.
Kasuga K, Yang R, Porter TF, et al. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol 2008; 181: 8677-87.
39.
Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 243-51.
40.
Khasawneh J, Schulz MD, Walch A, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci USA 2009; 106: 3354-9.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.