Heart failure (HF) is the leading cause of cardiovascular morbidity and mortality, and with its increasing prevalence, the healthcare systems worldwide face an HF pandemic [1–3]. Despite modern pharmacotherapy, including angiotensin receptor-neprilysin inhibitor (ARNI) and sodium-glucose cotransporter-2 inhibitors (SGLT2i), the prognosis of patients, especially those with advanced HF, remains poor [4].
Eicosanoids have been studied previously in basic cardiovascular and renal research. These metabolites of the cytochrome P-450 (CYP)-dependent epoxygenase pathway of arachidonic acid (AA), especially epoxyeicosatrienoic acids (EETs), importantly contribute to the regulation of cardiovascular and renal systems through their vasodilatory and natriuretic effect. Furthermore, in preclinical studies, they exert organ-protective actions [5–7]. Under physiological conditions, EETs are produced dominantly by endothelial cells (as endothelium-derived hyperpolarization factor – EDHF) and exhibit autocrine and paracrine action. EETs are rapidly transformed by soluble epoxide hydrolase (sEH) to biologically less active dihydroxyeicosatrienoic acids (DHETs) [5, 8] and excreted mainly by the kidney. The role of eicosanoids in human HF, however, remains unclear.
Our translational project (Eicosanoids in Human Heart Failure) aims to evaluate the role of eicosanoids in human HF. Here we present pilot data of the peripheral plasma levels of eicosanoids (14,15-EET and 14,15-DHET) measured by enzyme-linked immunosorbent assay (ELISA) in patients with HF in comparison to healthy controls.
Methods
A total of 50 consecutive adult patients with de novo or acute decompensated HF with reduced left ventricular ejection fraction (LVEF, HFrEF) admitted between July and September 2021 to our tertiary cardiovascular center were enrolled in this study; the only exclusion criterion was advanced renal failure before or during renal replacement therapy or a transplant program. The median age of the whole cohort was 70 years; 33% were women. As controls, we used blood samples of age- and sex-matched patients from our central hospital laboratory, where we excluded subjects with a diagnosis of HF in the medical records or with higher N-terminal pro B-type natriuretic peptide (NT-proBNP) in any of the blood tests available.
The blood samples were collected before breakfast in the morning the day after admission into tubes coated with microscopic silica particles as a clot activator. After centrifugation (10 min, 4000 rpm, 8°C), the serum was separated, aliquoted, directly analyzed (eicosanoid analysis) and the rest was stored at –80°C. The concentration of eicosanoids (14,15-EET and 14,15-DHET) was assessed using commercially available sandwich ELISA assays (MyBiosource, USA). Analyses were performed according to the manufacturer’s instructions. The sensitivity of 14,15 EET kit is 1 ng/ml, and the detection range is 3.12–100 ng/ml. Reproducibility – intra-assay CV (%) and inter-assay CV (%) are less than 15% [CV(%) = SD/mean ×100]. The sensitivity of 14,15 DHET was 0,1 ng/ml.
We compared HF patients’ plasma EET and DHET levels with controls and analyzed the potential correlations with age, sex, New York Heart Association (NYHA) class, NT-proBNP, estimated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (eGFR), and LVEF.
Clinical variables were summarized by a median or mean for continuous variables and by N (percentage) for categorical variables. Comparisons between two groups were performed using the two-tailed Student’s t-test or the Mann-Whitney U test as appropriate according to the Shapiro-Wilk normality test. The linear bivariate correlations were measured by the Pearson correlation coefficient (Pearson’s r). Plasma levels are presented by mean ± SD; p < 0.05 indicates a statistically significant difference (* in the figure). Statistical analysis was performed using SPSS Statistics 28 (IBM, Armonk, NY, USA), Prism 9 (GraphPad, San Diego, CA, USA) and Med.Calc 13 (MedCalc Software, Ostende, Belgium).
All participants provided written informed consent. The study protocol following the principles of the Declaration of Helsinki was approved by the University Hospital Motol ethics committee. ClinicalTrials.gov Identifier: NCT05305170 (Eicosanoids in Human Heart Failure).
Results
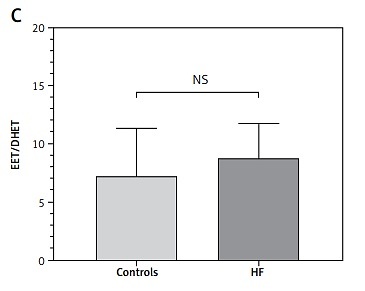
Both of the measured eicosanoids were significantly higher in patients with HF: 14,15-EET (91.3 ±25.7 ng/ml vs. 64.95 ±35.4 ng/ml, p < 0.001) (Figure 1 A) and 14,15-DHET (10.58 ±2.06 ng/ml vs. 9.07 ±1.60 ng/ml, p < 0.001) (Figure 1 B). The combination of peripheral levels of EET and DHET (i.e., total EET production) was also significantly higher in patients with HF (101.92 ±25.64 ng/ml vs. 74.02 ±35.92 ng/ml, p < 0.001), but the EET/DHET ratio, a parameter of EET degradation and decreased peripheral bioavailability of EETs (i.e., sEH activity), was not statistically different between the HF group and controls (8.75 ±3.0 vs. 7.24 ±4.06, p = 0.07) (Figure 1 C).
Figure 1
Peripheral plasma levels of 14,15-EET (A) and 14,15-DHET (B) in HF and control groups. The 14,15-EET to 14,15-DHET ratio (C)
In patients with HF, the mean NT-proBNP was 8840 ng/l, NYHA class III, eGFR 52.2 ml/min/1.73 m2, LVEF 38%, high-sensitivity C-reactive protein (hsCRP) 14.58 mg/l (Table I). The 14,15-EET levels did not correlate with age, NYHA class, NT-proBNP, eGFR, or LVEF, but there was a significant correlation with sex (r = –0.285, p = 0.013), with higher levels in men (89.26 ±31.39 ng/ml vs. 70.76 ±29.86 ng/ml, p = 0.013). Therefore, we compared men and women separately in HF and control groups with a significant intergroup difference (HF vs. controls) for men (93.88 ±27.99 ng/ml vs. 76.19 ±37.82 ng/ml, p = 0.016) and women (85.57 ±19.06 ng/ml vs. 55.96 ±31.84 ng/ml, p = 0.004). The 14,15-DHET levels did not correlate with age, sex, NT-proBNP, NYHA class, eGFR, or LVEF.
Table I
Patients’ characteristics
Discussion
In this proof-of-method part of our academic translational study, we found that peripheral plasma levels of both eicosanoids, active 14,15-EET and its inactive metabolite 14,15-DHET, were higher in patients with de novo or acute decompensated HFrEF compared to controls. The production of EETs was increased, but not their degradation to DHETs (EET/DHET ratio). The levels of 14,15-EET correlated with sex and were higher in men, both in the HF group and the control group.
One possible explanation could be higher production in the failing heart or peripheral tissue as a compensatory mechanism for tissue deficiency or decreased degradation either in the heart or in peripheral compartments. That needs to be elucidated by collecting blood samples from more body compartments (i.e. peripheral venous, peripheral arterial, central venous, central arterial and coronary sinus). So far, we can only extrapolate from our previous preclinical animal studies.
According to preclinical studies, the therapeutic potential of eicosanoids (due to their vasodilatory and natriuretic effect) seems promising in HF [5, 7]. Since EETs are rapidly transformed by sEH to biologically inactive DHETs, sEH inhibition was employed in most previous studies in which anti-hypertensive, cardio-, and renoprotective effects were reported [8]. However, this strategy might prove less successful whenever endogenous EET biosynthesis is compromised; thus, EET-agonistic analogs designed to resist degradation represent an alternative approach [9, 10]. In our recent animal study, we found that in a rodent volume-overload HF model induced by aortocaval fistula, the tissue EET deficiency in the left ventricle and kidney contributes to the poor prognosis and that pharmacological strategies that include EET analogs improve survival of this model by reducing bilateral cardiac eccentric hypertrophy and decreasing lung congestion [11].
The EET/DHET ratio is commonly used to assess the bioavailability of active metabolites [5, 6, 12], as validated in our preclinical study, where this ratio was reduced by around 65% in the kidney and left ventricle (LV) in an animal HF model when compared to controls. There were no significant differences in the kidney and LV protein expression of the enzymes responsible for EET production. However, the expression of sEH protein, an enzyme responsible for converting EETs to DHETs, was significantly increased in the animal HF model in LV tissue [11]. In this pilot human study, we did not measure the direct tissue concentrations of eicosanoids in the heart or kidney, where we expect the deficiency of active EETs according to our previous preclinical data [11].
It was proposed that intrarenal EETs may also act as an endogenous compensatory system opposing increased renin-angiotensin system (RAS) activity [12, 13]. Therefore, the increased peripheral concentration of eicosanoids might compensate for decreased availability in heart and renal tissue and countervail the activated RAS. Several body compartments contribute to the eicosanoid peripheral plasma levels because various cell types produce eicosanoids as paracrine and autocrine agents [5].
Any inflammatory diseases or steroid intake may influence the results of plasma eicosanoid levels, although this possible interaction has not been studied yet, especially in human HF. In our cohort, there was no patient or control on steroidal medication during blood collection, index hospitalization, or chronically. Although we found higher plasma hsCRP levels in the HF cohort than in controls (some of the HF decompensations were due to acute inflammatory diseases), there was no correlation between hsCRP and eicosanoid levels, suggesting no full explanation of our findings.
Since this was a pilot study to prove the feasibility of measuring the peripheral plasma levels of eicosanoids in HF patients by the ELISA method, the number of patients and controls is relatively low, limiting further analyses. We only enrolled de novo or acute decompensated HFrEF patients; thus, plasma eicosanoid levels of stable HFrEF patients and patients with only mildly reduced (HFmrEF) or preserved LVEF (HFpEF) remain to be elucidated in the next part of our project.
In conclusion, we found that levels of peripheral plasma eicosanoids (14,15-EET, 14,15-DHET) measured by ELISA are raised in patients with de novo or acute decompensated HFrEF compared to age- and sex-matched controls. These findings support further research into eicosanoids in HF.