Introduction
Stroke is the third leading cause of death and a major cause of disability worldwide. It is a focal cerebral insult that often results in neurological and medical sequelae with long-term implications for quality of life. Ischaemic stroke comprises the majority of strokes (85%), and haemorrhagic stroke accounts for the rest. Impairments associated with stroke can be motor, perceptual, sensory, cognitive, and psychological [1]. It is estimated that about 70% of stroke patients have upper limb residual impairments that compromise activities of daily living. The return of upper limb function has been identified as an important rehabilitation goal [2].
Hemiplegic shoulder pain (HSP) is considered one of the most debilitating complications following stroke [3]. Between 30% and 65% of stroke patients may experience HSP [4]. Pain can result from shoulder structural injury and abnormal posture, which may damage the surrounding tissues over time [5]. Impingement of rotator cuff, subluxation or capsulitis of glenohumeral joint, bicipital tendinitis, shoulder muscles spasticity, and shoulder hand syndrome are other causes of shoulder pain post stroke. Shoulder pain is demonstrated as a predictor for decreased arm functional adequacy, high levels of depression, and poorer quality of life [6].
A wide array of pathologies can potentially underly the development of HSP. Three possible mechanisms have been identified: soft-tissue lesions, impaired motor control (muscle tone changes), and altered peripheral and central nervous system activity. The pathomechanism is thought to be during the acute and subacute phase of stroke; flaccid paresis occurs resulting in potential subluxation of the shoulder, and/or imbalance of shoulder joint control and soft-tissue structure, resulting in altered mechanics of movement and increased susceptibility to injury. Spasticity causes abnormal scapulohumeral rhythm and leads to impingement of the rotator cuff or other structures in the subacromial space. Several predisposing factors are linked with HSP, including incorrect handling, trauma during post-stroke rehabilitation, passive abduction greater than 90°, or forced abduction without lateral rotation or immobilization of the affected limb [7, 8].
Ultrasonography (US) is an essential method in the evaluation of post-stroke HSP; it is superior to other imaging techniques that have been used in the evaluation of shoulder soft tissue structures. A variety of advantages are associated with it, including real-time imaging, direct multi-planar evaluation, cost-effectiveness, short examination time, the ability to compare side-by-side immediately, and the absence of ionizing radiation [9].
An extracorporeal shock wave is defined as a sequence of single sonic pulses characterized by high peak pressure (100 MPa), a fast rise in pressure (< 10 ns), and a short lifecycle (10 µs). A specific generator transfers the energy directly to the targeted region, with a flow density ranging from 0.003 mJ/mm2 to 0.888 mJ/mm2 [10]. Extracorporeal shock wave therapy (ESWT) can cause energy gradients and torsional tension between tissues of different densities through energy conversion and transmission, causing a cavitation effect that induces various biological effects. Many clinical studies have shown that shock waves are effective in the management of tendon and bone disorders; for example, pseudoarthrosis, shoulder tendinitis, plantar fasciitis, and many other tendon disorders, particularly among athletes [11].
Ultrasonography (USS) is advantageous over other imaging methods that have been used in evaluating the shoulder. Its advantages include broad availability, cost-effectiveness, real-time imaging, direct multiplanar assessment, immediate side-to-side comparison, short examination time, and lack of ionizing radiation [2]. Furthermore, it closely rivals shoulder magnetic resonance imaging (MRI) in accurately diagnosing full thickness rotator cuff tears [3, 4] and has been shown to be preferred to MRI by patients with shoulder pain.
Several studies have investigated the efficacy of using extracorporeal shock wave therapy on spasticity [12–16] and hemiplegic shoulder pain post stroke [10]. However, displaying the therapeutic possibilities of using extracorporeal shock wave therapy on the structural abnormalities that happen in the shoulder post stroke in relation to its effect on the level of shoulder pain and disability index post stroke have not been well defined in any previous studies.
Material and methods
Design of the study
A single-blind randomized controlled study was administrated in an outpatient private clinic from September 2020 to October 2021. Prior to enrolment an informed consent form was signed by each participant. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice standards, and it was approved by the Ethics Committee for clinical research of the Faculty of Physical Therapy, Cairo University with registration number (P.T.REC/012/002870). The study has an identification number on ClinicalTrials.gov: NCT04859673.
Confidentiality
All research procedures were carried out in a closed room in a private outpatient clinic, from the orientation to the actual application of the study. During the study, we made sure that only the investigators were in the room.
Declaration of interests
Other than the information and data acquired from participants for the research study, there is no interest.
Access to data
The patient’s personal data and details were recorded in an MS Excel spreadsheet that was only accessible to the research investigators. The spreadsheet was password protected and kept on the principal investigator’s PC.
Ancillary and post-trial care
There were no physical, psychological, or social dangers in this study. If subjects report any unexpected symptoms during treatment, the trial could have been halted.
Participants
The study was conducted on 30 subacute stroke patients with hemiplegic shoulder pain. All patients were clinically diagnosed and referred from a general neurologist. Computed tomography scan and MRI were used to confirm the diagnosis of stroke in the territory of the middle cerebral artery.
Patient’s ages were between 40 and 60 years, with mild to moderate upper limb spasticity (grade 1+: 2) according to the modified Ashworth scale (MAS). All conducted patients experienced a single stroke during the last 3 months with cognitive capacity that enable them to comprehend and follow the instructions (Mini-Mental Scale score > 24).
Exclusion criteria included patients with a history of shoulder pain, trauma, or surgery prior to stroke, patients taking warfarin with an international normalized ratio above 4.0, oral NSAIDs 3 days prior to study, or intra-articular injections 1 month prior to study. Patients who are unable to express their own pain severity, those who had a cardiac pacemaker, and those who were osteoporotic were also disqualified.
Randomization
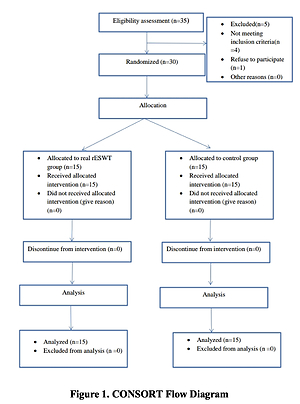
Thirty-five subacute stroke patients with shoulder pain were initially evaluated for eligibility. Four patients did not meet our inclusion criteria, and one refused to participate. Allocation of the remaining 30 patients took place randomly via computer-generated random numbers into 2 groups of equal size. The examiners were blinded to which group the patients belonged (CONSORT Flow Diagram (Figure 1)).
Outcome measures
Primary: shoulder structural abnormalities
A Samsung HS70A (Samsung Medison, Seoul, Korea) ultrasonography machine was used to assess the structural changes of the hemiplegic shoulder. Ultrasonography procedures were applied while the patient was seated on a chair, and the affected shoulder was examined in all patients via scanning 4 main tendons: biceps brachii (long head), supraspinatus, infraspinatus and subscapularis, and glenohumeral joint, as well as the existence of adhesive capsulitis (by measuring the coracohumeral ligament thickness) [17]. Abnormal ultrasound (USS) findings were assigned as follows: a) Full-thickness tear of rotator cuff presented as a full-thickness hypoechoic defect, visible hyaline cartilage, or appearance of heterogeneous hypoechoic cuff due to deltoid or subacromial-subdeltoid bursa herniation into the cuff. b) Partial-thickness tear of cuff showed as hypoechoic defect within the tendon (more than 3 mm) or in an articular or bursal surface that appears in both planes (transverse and longitudinal). c) Tendinosis was presented as diminished echogenicity with tendon enlargement (> 8 mm for biceps and > 2 mm for rotator cuff). d) Long head of biceps tendon (LHBT) sheath effusion was defined as an anechoic region (> 2 mm) encircling the LHBT in transverse view or an anechoic/hypoechoic crescent penetrating the LHBT in the longitudinal views. e) Bursitis was diagnosed by the presence of fluid in the subacromial-subdeltoid (SASD) bursa with a thickness of more than 2 mm. f) Shoulder subluxation was diagnosed by increase in the distance between acromion and greater tuberosity by more than 0.4 cm over the normal value (1.91–2.84 cm). g) Adhesive capsulitis was identified by increased thickness of the coraco-humeral ligament of more than 3 mm [17, 18].
Scoring: If an abnormal USS finding was present, it was given a score of one (1), and if it was not present, it was given a score of zero (0). So, the presence of LHBT effusion, SASD effusion, subluxation, or adhesive capsulitis all received one point each. The 4 tendons were evaluated individually per shoulder and were graded identically for tendon tear, tendinosis, or degeneration. The combination of these results produced a raw USS score, with the lowest score being zero (normal examination) and the highest score being 16. The raw USS scores were then divided into graded USS scores: (0) for normal shoulder, (1–2) for mild damage, (3–4) for moderate damage, (5–6) for severe damage, and (> 6) for intense damage [8]. All measurements were taken at baseline and post intervention for both groups.
Secondary: the pressure-pain threshold
The pressure-pain threshold (PPT) is the lowest pressure at which the pressure sense is perceived as a pain. Pressure algometry is a valid and reliable method to evaluate deep somatic tissue sensitivity. A hand-held Baseline® Dolorimeters algometer was used to assess the pressure-pain threshold (PPT). It has a pistol-style grip and a rod at the tip with a pressure-sensitive gauge strain. The algometer tip was positioned on the middle of the deltoid muscle on the affected shoulder of each patient while seated. Pressure was conducted at a rate of 1 kg/cm2/s using a 0.785 cm2 rubber tip. As the algometer was pressed, the force gradually increased, the algometer display was not visible to the patients at any time, patients were instructed to say “stop” once they felt pain, after which the device was released, and the force was measured. The threshold was assessed 3 times for each patient at each site, and the average was calculated and utilized for analysis [19].
Shoulder pain and disability index (SPADI)
This is a self-administered 13-item questionnaire that involves 2 main domains: pain and functional activities. The pain domain consists of 5 questions regarding the severity of pain, and functional activities are assessed with 8 questions designed to measure the degree of difficulty experienced in activities of daily living that require upper-extremity use. Items of the questionnaire were demonstrated clearly, then patients were asked to place a mark on a 10-cm visual analogue scale for each question from (right end) indicating “worst pain imaginable/so difficult required help” to (left end) “no pain/no difficulty”. Scores were demonstrated as follows: a maximum of 50 points for the pain subscale, 80 points for the disability subscale, and 130 points for the total index score. All scores were expressed as a percentage. Final score of each part was statistically analysed separately [20].
Intervention
Radial extracorporeal shock wave therapy
The shock wave was delivered using a Swiss DolorClast® Master – shockwave therapy system machine. It is equipped with a master console, external compressor, 15-mm contact head, and a handpiece. Each patient was instructed to sit comfortably with the affected shoulder exposed. The stimulator head with the transmitter was applied to greater and lesser tuberosities of the humeral head to activate the rotator cuff tendons and the shoulder capsule. These stimulation points corresponded to the insertion points of the subscapularis and supraspinatus muscles. The subscapularis insertion site was stimulated by externally rotating the shoulder and flexing the elbow at 90°, while the supraspinatus insertion site was stimulated by internally rotating the shoulder and slightly extending the elbow. Each stimulation site received 3000 pulses (1500 pulses/site) at a submaximal pressure of 0.39 to 1.95 mJ/mm2 and a frequency of 12 Hz each session, according to the patient’s tolerance for pain without the use of local anaesthetics [10].
Designed physical therapy program for shoulder
It included a Bobath approach for the upper limb, scapular mobilization, stretching exercises for inward rotators, passive range of motion, and active assisted exercises for shoulder movements [5].
Patients in the real rESWT group (GA) underwent rESWT plus a designed physical therapy program for the shoulder joint. The duration of session was about 75 min (15 min for rESWT and 60 min for the physical therapy program) according to the patient’s tolerance. Patients in the control group (GB) received a sham rESWT plus the same designed program of physical therapy as in (GA). The sham rESWT was done by applying the stimulator head to the same stimulation points; because the transmitter had been removed, stimulation was not provided, and the patients got only air pressure and sound at the same frequency [10]. The treatment protocol for both groups was 2 times per week for a whole month. Intervention (rESWT + physical therapy program) was applied by a therapist, and to avoid bias, assessment of the SPADI scale and PTT was done by another therapist.
Sample size estimation
Prior to the study administration, the sample size was calculated using G*power 3.0.10 software (Heinrich Heine University Düsseldorf, Düsseldorf, Germany). It was calculated based on previous studies (F test MANOVA: Repeated measures, within- between interaction, α = 0.05, effect size = 0.35, power = 0.95%, and large effect size), which showed that the suitable size for this study was approximately 30 patients (15 patients for each group).
Statistical analysis
Data analyses were conducted using SPSS Version 21 (SPSS Inc., Chicago, USA). Distribution of gender and degree of spasticity in both groups were evaluated using the χ2 test. The Shapiro-Wilk test was used to test the normality of data. Mann-Whitney and Wilcoxon tests were used to compare USS score pre and post treatment between and within groups, respectively. 2×2 multivariate analysis of variance (MANOVA) was used to analyse SPADI and PPT variables, with the treatment groups (GA versus GB) used as between subjects’ factor and time of assessment (pre and post treatment) used as within subjects’ factor. The independent t-test was used to compare age and duration of illness between both groups. For all statistical examinations the level of significance was p < 0.05.
Results
There was no statistically significant difference between both groups regarding age, sex, degree of spasticity, and duration of illness (p > 0.05) (Table I).
Table I
Comparison of general characteristics of patients in both groups
The statistical analysis of measured variables pre and post treatment within each group revealed a significant reduction of all SPADI scores (pain, disability, and total scores) in both groups (p < 0.05): the percentage of changes were 41.58, 36.94, and 38.99 in GA compared to 10.76, 12.23, and 11.05 in GB, respectively, while there was a significant reduction of USS score and increase of PPT only in GA (p = 0.001) (Tables II and III).
Table II
Comparison of USS scores within and between both groups
| Variable | Real rESWT (GA) group Median (IQR) | Control (GB) group Median (IQR) | P-value | |
|---|---|---|---|---|
| USS score | Pre-treatment | 4 (1) | 3 (0) | 0.1281 |
| Post-treatment | 1 (1) | 3 (0) | 0.0001* | |
| P-value | 0.0001* | 0.99 | ||
Table III
Comparison of SPADI and PPT variables within and between both groups
| Variable | Real rESWT (GA) group Mean ± SD | Control (GB) group Mean ± SD | P-value | |
|---|---|---|---|---|
| SPADI pain score | Pre-treatment | 91.53 ±5.08 | 94.13 ±3.96 | 0.129 |
| Post-treatment | 53.47 ±11.62 | 84.00 ±3.68 | 0.001* | |
| P-value | 0.001* | 0.001* | ||
| SPADI disability score | Pre-treatment | 89.07 ±3.97 | 92.76 ±6.16 | 0.061 |
| Post-treatment | 56.17 ±12.88 | 81.42 ±5.77 | 0.001* | |
| P-value | 0.001* | 0.001* | ||
| SPADI total score | Pre-treatment | 90.31 ±3.25 | 92.62 ±5.38 | 0.165 |
| Post-treatment | 55.10 ±11.64 | 82.39 ±4.12 | 0.001* | |
| P-value | 0.001* | 0.001* | ||
| Pain pressure threshold (PPT) | Pre-treatment | 1.29 ±0.2 | 1.46 ±0.28 | 0.07 |
| Post-treatment | 3.23 ±0.61 | 1.77 ±0.58 | 0.001* | |
| P-value | 0.001* | 0.057 | ||
The between-group analysis revealed no significant differences between groups before treatment (at the baseline assessment) in all measured variables (p > 0.05). While post-treatment between-group analysis was as follows: significant reduction in all SPADI scores (pain, disability, and total scores), USS score, and significant increase of PPT in favour of GA (p < 0.05) (Table II and III).
Discussion
This study provides evidence on the efficacy of adding radial extracorporeal shock wave therapy (rESWT) to a designed physical therapy program for shoulder structural abnormalities as well as the pain and disability level in subacute stroke patients. Approximately one-third of all stroke patients experience shoulder pain that has a negative impact on daily activities and quality of life. To the best of our knowledge, this study was the first randomized controlled trial that investigated the efficacy of rESWT on shoulder structural abnormalities in accordance with pain and disability levels in subacute stroke patients.
The findings of the current study revealed a significant reduction in the ultrasonographic structural abnormalities of the hemiplegic shoulder in the real rESWT group. This may be attributed to the therapeutic effect of rESWT, which facilitates tendon healing by increasing angiogenesis, promoting tenocyte proliferation and collagen metabolism, increasing collagen turnover, and enhancing neovascularization of injured tissue, which is accompanied by a release of endothelial nitric oxide, vascular endothelial growth factor, and proliferating cell antinuclear antigen [21]. Another assumption that supported our results was an investigation into the role of shock wave therapy in resolving oedema and reducing inflammatory cytokines in injured tendons as well as its role in increasing the expression of growth factors like TGF-1 and TGF-1, which play an integral role in tissue regeneration and tendon repair [22].
The results of the current study also agree with those of Pan et al. [23], who concluded that 2 sessions of rESWT 14 days apart showed great therapeutic outcomes in tendinitis of the shoulders, especially of the rotator cuff with arc-type calcific plaques. However, the outcomes of our study are more thorough, because the therapeutic efficacy of rESWT on the shoulder structural changes were examined via ultrasonographic scanning of 4 main tendons: biceps brachii (long head), supraspinatus, infraspinatus and subscapularis, and glenohumeral joint, as well as the existence of adhesive capsulitis. Also, our findings may be correlated with the doubled number of rESWT sessions applied in our study.
The presented study is in accordance with other previous studies that provided evidence about the positive effect of rESWT on relieving pain by increasing the pain pressure threshold and decreasing the SPADI pain score in a real rESWT group. This could be attributed to the direct suppressive effects of shock waves on nociceptors, as well as the hyper-stimulation mechanism that disables the gate control mechanism. Other studies declared that shock waves helped to reduce pain by lowering substance P levels in the target tissue and decreasing substance P synthesis in the dorsal root ganglia [24, 25]. Furthermore, some studies have described the efficacy of shock waves in reducing the expression of inflammatory mediators at high levels (matrix metalloproteinases and interleukins). In addition, it can boost regional blood flow and reduce muscular tension and stiffness as well as pain by interfering with the flow of excessive activation of nociceptors and selective destruction of nonmyelinated fibres [26].
Regarding the efficacy of rESWT on pain, the findings obtained in our study revealed a significant reduction in the level of pain by the end of the treatment protocol; this is congruent to the results found by Kim et al. [10], who concluded that rESWT is an effective and safe modality for pain management in people with hemiplegic shoulder pain. However, the method used to evaluate pain in our study was more objective than that used by Kim et al. because we used PPT instead of the visual analogue scale. Another advantage to our study is the frequency of application because the optimum treatment protocol for rESWT is 3 sessions per week [27]. In our study rESWT was applied twice per week for 4 weeks compared to 4 times a week for 2 weeks as applied by Kim et al.
In terms of the functional improvement in the shoulder after rESWT, the results of the present study revealed a reduction in all SPADI scores (pain, disability, and total scores) for both groups, with a more pronounced effect in favour of the real rESWT group (GA). This may be attributed to the anti-inflammatory and anti-fibrotic effect of rESWT, which helped to reduce the synovial inflammation and capsular fibrosis, with a consequent improvement in shoulder ROM and function [28].
In this study, the designed physical therapy program for the shoulder, including Bobath, ROM, stretching, and scapular mobilization, showed improvement in the pain level and functional abilities for both groups; this may be attributed to improved blood flow to the affected areas, reduced adhesions, modulated spasticity, and inhibition of nociceptors [29–31]. The effect of exercise was discussed in a previous comparative study conducted by Engebretsen et al. [32]; they investigated the effect of supervised exercises versus rESWT on subacromial shoulder pain in stroke patients with one-year follow-up. The results revealed reduction of SPADI score and pain, and improvement of shoulder function in both groups with no significant difference between them. Also, there was a recommendation on the short-term efficacy of supervised exercises (SE) rather than rESWT. In our study, we assessed the efficacy of adding rESWT to a designed physical therapy program, and there was significant difference in the pain, SPADI, and functional scores in favour of the real rESWT group. This may be attributed to the cumulative effect of rESWT and the designed physical therapy program, in addition to the doubled number of rESWT sessions used in our study.
On the other hand, different findings have been reported by other authors: Speed et al. [33] claimed that there is no evidence of an added benefit from rESWT compared with sham treatment. This contradiction may be attributed to different sites of stimulation, number of sessions, and session intervals between the 2 studies. Our findings also contradicted with Kvalvaag et al. [34], who concluded that rESWT has no additional effect on the supervised exercises in the management of subacromial shoulder pain after 24 weeks, except in the subgroup of patients with calcification in the rotator cuff. The discrepancy between the 2 studies may be due to different durations of treatment protocol – the study of Kvalvaag et al. applied rESWT once a week for 4 weeks, while in our study rESWT was applied 2 times a week for 4 weeks.
In this study, post-treatment changes in USS score and PPT in the control did not reach a significant difference, which may be due to the short treatment period.
It is necessary to point out some limitations in this study. This study was limited by only reporting the immediate post-treatment effect of rESWT, so we cannot generalize about the long-term efficacy of rESWT after stopping our program. Also, the included patients were limited to subacute hemiplegic with shoulder pain, so further research is recommended to investigate the impact of rESWT in patients with different hemiplegic onsets such as acute and chronic hemiplegic shoulder pain.
Further studies are recommended on a larger sample size, and the effect of gender should be considered in future work. Future researchers should also address various age groups in their sample and incorporate different follow-up periods in their study design. We would also recommend the use of NIHSS in future work in comparative studies to assess the effect of rESWT on HSP in subacute stroke patients with different stroke severities.
In conclusion, the use of the radial extracorporeal shock wave therapy as an adjunct to a designed physical therapy program has a significant effect on reducing shoulder structural abnormalities and consequently pain and disability in subacute stroke patients.