1. Preamble. Why do we need new guidelines in 2021?
Despite 30 years (simvastatin was approved for clinical use by the FDA in 1991 [1]) of experts’, societies’, and patient organisations’ efforts, lipid disorders still comprise a diagnostic and, before all, therapeutic challenge. This applies to adequate risk assessment in patients, introduction of appropriate treatment, problems with patient compliance, as well as to problems with so important non-pharmacological treatment – diet, body mass reduction, or regular exercise [2]. The significance of therapeutic inertia, either in the form of inadequate therapy (usually the lack of intensive statin treatment or, even less likely, combination therapy) or evident errors such as dose reduction or cessation of treatment following achievement of the therapeutic target, also cannot be diminished. That is why in Poland there are still nearly 20 million individuals with hypercholesterolaemia, most of them unaware of their condition [3]; that is why only ca. 5% of patients with familial hypercholesterolaemia out of predicted as much as 140,000 have been diagnosed; that is why other rare cholesterol metabolism disorders are so rarely diagnosed in Poland [4, 5].
Let us hope that these guidelines, for the first time being an effect of work of experts representing 6 scientific societies, as well as the network of Polish Lipid Association (PoLA) lipid centers (currently under development, https://ptlipid.pl/siec-centrow-lipidowych) (the list of centers is presented in Appendix), being a part of the European Atherosclerosis Society (EAS) European lipid centers, certification of lipidologists by PTL, or the growing number of centers for rare diseases, with a network planned by the Ministry of Health, improvements in coordinated care for patients after myocardial infarction (KOS-Zawał) associated with the need of lipid profile assessment at specific time points, reimbursement of innovative agents (after PCSK9 inhibitors, hopefully also inclisiran, bempedoic acid, evinacumab, and pelacarsen), as well as introduction in Poland of an effective (at least) primary prevention program, will make improvement in relation to these unmet needs in diagnostics and treatment of lipid disorders possible.
Moreover, over the last few years the approach to treatment of patients with a high cardiovascular risk has totally changed from treatment aimed at a specific risk factor (i.e., glucocentricity or lipo-centricity) to effective diagnostics, monitoring, and treatment of all risk factors and general cardiovascular risk as well as concomitant diseases, stressing the role of residual risk, and the intensity of the applied therapy. In the case of lipid disorders, intensive lipid-lowering treatment is currently recommended (and not just intensive statin treatment, as it has been for years) in order to achieve in our patients as low concentrations of low density lipoprotein (LDL) cholesterol as possible, according to the rule of “the lower the better”, but also to do it as soon as possible (“the earlier the better”) and maintain it as long as possible (“the longer the better”), as this gives us a chance to reduce the risk of cardiovascular events even in every other patient (50–55%) [6, 7].
Taking into consideration immense challenges still present in diagnostics and therapy of lipid disorders, changes in the approach to treatment, including innovative molecules, as well as the most recent results of numerous studies (lipidology and atherosclerosis research are currently the most rapidly developing specialty in medicine), Polish Lipid Association (PoLA), along with College of Family Physicians in Poland (CFPiP), Polish Cardiac Society (PCS), Polish Society of Diabetology (PSD), Polish Society of Laboratory Diagnostics (PSLD), and Polish Society of Hypertension (PSH), decided to prepare comprehensive guidelines concerning management of lipid disorders, with special attention paid to the practical aspect of these guidelines, as we would like to make them an actual tool for everyday work with patients suffering from lipid disorders.
2. Introduction
Although 5 years elapsed since the time of publication of the previous guidelines, lipid metabolism disorders remain the most common and the worst controlled cardiovascular risk factor in Poland [8]. Along with tobacco smoking, type 2 diabetes mellitus, arterial hypertension, improper dietary habits, and insufficient physical activity leading to overweight and obesity, they are the primary modifiable risk factors of atherosclerosis and its most important complications, such as ischaemic heart disease, cerebral stroke, and peripheral artery disease [9].
The results of epidemiological studies performed in our country indicate that their prevalence grows constantly due to spreading of unhealthy dietary habits and sedentary lifestyle resulting in an epidemic of overweight and obesity [10], in which the coronavirus pandemic also played a very detrimental role in the last 2 years. Recent results of large observational studies by Non-Communicable Disease Risk Factor Collaboration (NCD-RisC) indicate that Poland belongs to countries in which the least changes in mean total cholesterol or non-high density lipoprotein (non-HDL) cholesterol concentration may be observed, even with trends indicating their growth in men in subsequent years, which, unfortunately, has translated into a slight decrease or the lack of reduction of mortality due to ischaemic heart disease and ischaemic stroke dependent on this risk factor in the years 1990–2017 [11, 12].
Therefore, their control, with particular emphasis on lipid disorders, remains one of the main public health challenges, also in the present context of struggle to improve the health of Poles in the post-pandemic era. To face these challenges, comprehensive preventive activities at the population level are needed, especially those concerning primary prophylaxis, which should be concentrated on selection of high-risk patients, adequate widespread health education, and optimum treatment (including non-pharmacological interventions), to avoid or delay development of ischaemic heart disease, stroke, or peripheral artery disease.
Family physicians, as well as other healthcare professionals (cardiologists, diabetologists, internists, nurses), bear a special responsibility with respect to high-risk patients, i.e., the group to which numerous patients with dyslipidaemia belong. This common, well-organised struggle, with good communication between family physicians and specialists (which is still often missing), should be an element of a wider strategy aimed at reduction of the total cardiovascular risk, and ultimately at reduction of mortality, morbidity, and disability due to cardiovascular disease.
3. Development of the Guidelines
Members of the Steering Committee who prepared these guidelines were selected and indicated by Polish Lipid Association (PoLA), College of Family Physicians in Poland (CFPiP), Polish Cardiac Society (PCS), Polish Society of Diabetology (PSD), Polish Society of Laboratory Diagnostics (PSDL), and Polish Society of Hypertension (PSH) as experts in treatment of patients with lipid disorders. The Steering Committee has carefully reviewed published evidence on the management of dyslipidaemia, including its diagnosis, treatment, and prevention, as well as critical evaluation of diagnostic and therapeutic procedures, including benefit-risk assessment and cost-effectiveness indicators. The level of evidence and the strength of recommendations for each intervention were weighed and categorised using widely recognised defined classifications presented in Tables I and II. As these guidelines are intended to be a practical tool, apart from application of the appropriate class and strength of recommendation, each chapter is additionally independently summarised, pointing to the information necessary to remember by physicians and key points of recommendation, in terms of their application in everyday clinical practice.
Table I
Classification of recommendations in the guidelines
Table II
Level of evidence
Experts being members of the Writing Committee submitted the declaration of interest forms regarding all associations that could be perceived as actual or potential sources of conflict of interest (see details at the end of this document). After final approval of their content, the final pre-print version of the guidelines will be published immediately on the webpages of the relevant societies and then, if possible, simultaneously published in the Archives of Medical Science (indicated by PoLA), Lekarz Rodzinny (official journal of CFPiP), Kardiologia Polska (Polish Heart Journal, PCS), Diagnostyka Laboratoryjna (Laboratory Diagnostics, PSDL), Current Topics in Diabetes (PSD), Nadciśnienie Tętnicze w Praktyce (PSH) and additionally Lekarz POZ to reach as many interested parties as possible.
Family physicians and physicians of other specialities involved in the care of patients with lipid disorders are encouraged to take these guidelines into full consideration in clinical evaluation as well as in development and implementation of medical strategies for prevention, diagnostics, or treatment. However, these guidelines do not in any way disclaim the individual responsibility of physicians for making appropriate and accurate decisions, taking into account the condition of a specific patient, and following consultation with the patient and, if necessary, with the patient’s caregiver. Healthcare professionals are also responsible for verification of the rules and regulations concerning medicines and devices at the time of their prescription/application.
4. Epidemiology of lipid disorders in Poland
Disorders of lipid metabolism are the most common cardiovascular risk factor; this has also been confirmed in Polish screening studies [4, 10]. Despite continuous education of physicians and patients and availability of different lipid-lowering therapies, the effectiveness of detection and treatment of dyslipidaemia in Poland remains unsatisfactory. Over the last nearly 40 years, numerous, extensive studies have been conducted in Poland to evaluate the prevalence of dyslipidaemia. A summary of the most important studies concerning lipid disorders, including the method of patient sample selection and the years of their conduction, is presented in Table III.
Table III
Summary of Polish epidemiological studies on dyslipidaemia according to the method of patient sample selection
Depending on the sample selection method, the prevalence of dyslipidaemia in Poland is estimated at 60–80% of people in the population over 18 years of age [13]. The first data on the prevalence of hyperlipidaemia (the Pol-MONICA study) indicated hypercholesterolaemia in just over 70% of women and nearly 73% of men [14]. In that study, the percentage of individuals with the low-density lipoprotein-cholesterol (LDL-C) concentration above the normal range was higher in men (60%) than in women (53%) [14]. Decreased HDL-C concentration was observed in nearly 2% of women and 10% of men, while elevated triglyceride (TG) concentration was observed in 6% of women and 21% of men [14]. In another study (SPES – Southern Poland Epidemiological Survey) hypercholesterolaemia was reported in nearly 56% of the subjects (58% of women and 52% of men, respectively) [15]. The cited results, however, were not nationwide but restricted to the ex-voivodeships of Warsaw and Tarnobrzeg (the Pol-MONICA study), and Katowice and Bielsko-Biala (the SPES study).
Further data on the prevalence of dyslipidaemia in Poland came from two nationwide studies with random sampling: the NATPOL III PLUS study and the WOBASZ study. The prevalence of hypercholesterolaemia was estimated in the NATPOL study at 59.5% in men and 62% in women, while in the WOBASZ study it was 67% and 64%, respectively [16, 17]. In the NATPOL study, the observed percentage of patients with the LDL-C concentration above the normal range was almost the same in men and women (55%) [16]. In the WOBASZ study, elevated LDL-C concentration was observed in 60% of men and 55% of women [17]. The proportion of patients with decreased HDL-C concentration in the NATPOL study was 17% and 6% in men and women, respectively, and in the WOBASZ study, 15% and 17%, respectively [16, 17]. The NATPOL study results showed that in Poland hypertriglyceridaemia occurred in 30% of the subjects, more often in men than women (38% vs. 23%) [16]. In the WOBASZ study, elevated TG concentration was observed in 31% of men and 20% of women [17].
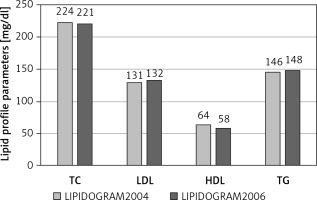
Significant prevalence of lipid disorders, estimated according to the US NCEP-ATP III (National Cholesterol Education Program – Adult Treatment Panel III) guidelines, was also observed in the nationwide studies LIPIDOGRAM2003, LIPIDOGRAM2004 and LIPIDOGRAM2006, in which a total number of nearly 49,000 primary health care (PHC) patients were eventually included in the databases (including LIPIDOGRAM 2015) [18–20]. In the LIPIDOGRAM2003 study, the mean values of lipid profile parameters were: total cholesterol (TC) – 228 mg/dl (5.9 mmol/l), LDL-C – 140 mg/dl (3.6 mmol/l), HDL-C – 57 mg/dl (1.5 mmol/l), and TG – 156 mg/dl (1.8 mmol/l) [18]. Hypercholesterolaemia > 200 mg/dl (> 5.2 mmol/l) was reported in 72% of the subjects, more often in women than in men (76% vs. 67%). Elevated LDL-C concentration (according to the normal values accepted at the time) of > 160 mg/dl (4.1 mmol/l) occurred in 28% of the enrolled subjects, more often in women than in men (30% vs. 24%). Decreased HDL-C concentration < 40 mg/dl (1.0 mmol/l) was observed in 6% of the subjects, 12% and 3% in men and women, respectively, while elevated TG levels > 200 mg/dl (2.3 mmol/l) were observed in 22% of the subjects, more often in men than in women (26% vs. 19%) [18]. Mean values of specific lipid profile parameters and the percentage of abnormal values in subsequent studies – LIPIDOGRAM2004 [19] and LIPIDOGRAM2006 [20] – are presented in Figure 1.
In another study, i.e. NATPOL 2011, mean values of lipid profile parameters in men and women, respectively, were: TC – 197.1 mg/dl (5.1 mmol/l) and 198.6 mg/dl (5.1 mmol/l), LDL-C – 123.6 mg/dl (3.2 mmol/l) and 123.7 mg/dl (3.2 mmol/l), HDL-C – 45.8 mg/dl (1.2 mmol/l) and 54.1 mg/dl (1.4 mmol/l), and TG – 140.9 mg/dl (1.6 mmol/l) and 104.0 mg/dl (1.2 mmol/l). The percentages of subjects with abnormal values were: TC > 190 mg/dl (4.9 mmol/l) – 54.3% (54.3% of men and 54.4% of women), LDL-C > 115 mg/dl (3.0 mmol/l) – 57.8% (58.3% of men and 57.3 of women), HDL-C < 40 mg/dl (1.0 mmol/l) in men – 32.5%, HDL-C < 45 mg/dl (1.2 mmol/l) in women – 22%, TG > 150 mg/dl (1.7 mmol/l) – 21.1% (28.4% of men and 14.0% of women) [4]. In the WOBASZ II study conducted 2 years later, hypercholesterolaemia was reported in 70.3% of men and 64.3% of women aged > 20 years (67.1% of the overall study population), while hypertriglyceridaemia with normal cholesterol concentration was observed in 5.6% of men and 2.4% of women [21]. Decreased HDL-C with normal TC and TG concentration was observed in 5.1% of men and 7.3% of women. Overall, at least one type of dyslipidaemia was reported in as much as 81.0% of women and 74.0% of women. As much as 60.6% of subjects with hypercholesterolaemia were not aware of this fact, and only 6% of patients were effectively treated and achieved reference values of lipid profile parameters [21].
In a 5-year nationwide prospective cohort study of LIPIDOGRAM 5-YEARS, conducted in the years 2004–2010 in a population of PHC patients treated for dyslipidaemia (n = 1841), the reduction of lipid profile parameter values was ineffective in nearly 50% of individuals with TC and LDL-C concentration above the normal range and in more than 30% of individuals with elevated TG levels [22–24]; this de facto confirmed the lack of improvement of effectiveness of treatment of lipid disorders observed also in the earlier screening studies of LIPIDOGRAM2004 and LIPIDOGRAM2006 [22–24].
Insufficient control of hypercholesterolaemia was also observed in subsequent studies, including a study in patients hospitalised for ischaemic heart disease followed up in the Krakow Ischaemic Heart Disease Secondary Prevention Programme [25]. The target LDL-C concentration of < 70 mg/dl (1.8 mmol/l) was observed in only 28.1% of patients, while in 71.9%, 38.6%, 24.4% and 10.3%, LDL-C was ≥ 70 mg/dl (1.8 mmol/l), ≥ 100 mg/dl (2.5 mmol/l) ≥ 115 mg/dl (3.0 mmol/l), and ≥ 160 mg/dl (4.0 mmol/l), respectively [25]. Moreover, what is still a major challenge for physicians and researchers, studies performed in the past twenty years suggest that the percentage of patients with ischaemic heart disease achieving the recommended LDL cholesterol concentration has not significantly changed as compared to the end of the 20th century (regardless of differences with respect to the recommended target LDL-C concentration) [26]. Interestingly, the percentage of patients achieving the target LDL-C concentration among those who declared treatment in PHC clinics did not differ significantly from the same percentage in patients who declared that their treatment was decided by a physician in a specialist cardiology clinic [26].
In the most recent nationwide study on the prevalence and treatment of dyslipidaemia in primary care patients (n = 13,724), i.e., LIPIDOGRAM2015 & LIPIDOGEN2015, conducted in the years 2015–2016, the results obtained did not indicate any significant improvement in the epidemiological situation concerning lipid disorders in Poland discussed above [10, 27, 28]. The results of the LIPIDOGRAM2015 study showed an elevated total cholesterol level of > 190 mg/dl (4.9 mmol/l) in 58% of active PHC patients > 18 years of age; LDL-C concentration > 115 mg/dl (3.0 mmol/l) was observed in 61% of the subjects, while decreased HDL-C concentration < 40 mg/dl (1.0 mmol/l) in men and < 45 mg/dl (1.2 mmol/l) in women was observed in 14% of the subjects [27, 28]. Elevated TG concentration > 150 mg/dl (1.7 mmol/l) was observed in 33% of patients. Mean values of lipid profile parameters in the overall population as well as in patients treated and not treated due to lipid disorders, depending on the prevalence of cardiovascular disease (CVD), are presented in detail in Table IV [10].
Table IV
Mean values of lipid profile parameters in patients with cardiovascular disease (CVD) and without CVD in the LIPIDOGRAM2015 study population
Despite changes in the prevalence of cardiovascular diseases and their risk factors (including lipid disorders) observed in Poland between the year 1990 and 2017, differences between Poland and Western Europe remain very high [29]. In Poland, as in other European countries, there are still discrepancies between the current clinical guidelines (2020) and clinical practice with respect to diagnostics and treatment of lipid disorders – only one in 3 patients in Europe and one in 4 in Poland achieves therapeutic goal; only 18% of patients in Europe, 17% in Poland, and only 13% in Central and Eastern European countries achieve the therapeutic goal for very high-risk patients (< 55 mg/dl/< 1.4 mmol/l), not to mention extreme risk patients, of whom less than 10% achieve their therapeutic goal (< 40 mg/dl/< 1 mmol/l) [30, 31].
It is also worth mentioning that, according to calculations based on predictions from the studies discussed above, in Poland there may be as many as 140–150 thousand patients with familial hypercholesterolaemia (predicted prevalence of 1 : 250]) [32, 33]. Unfortunately, only less than 5% of them are diagnosed despite existence of the registries, i.e., the Gdansk registry and the PTL registry, as well as a therapeutic programme for patients with FH in the context of treatment with PCSK9 inhibitors. Based on the TERCET Registry, it was observed that the prevalence of probable/certain FH diagnosis and possible FH diagnosis was 1.2% and 13.5%, respectively, and in patients with acute coronary syndrome (ACS) 1.6% and 17.0%, respectively [34]. The 30-day mortality rate was higher in patients with certain and probable FH diagnosis than in patients without FH (8.2% and 3.8% vs. 2.0%, respectively). Similar results were observed (using the Propensity Score analysis) for all-cause mortality at 36 and 60 months as compared to patients without FH (11.4% vs. 4.8 % and 19.2% vs. 7.2%, respectively) [34].
KEY POINTS TO REMEMBER
Assuming that in a model practice a family physician takes care for a population of about 2,500 patients, of which adults account for more than 75%, and considering the prevalence of dyslipidaemia in Poland estimated at 60–80% in individuals over 18 years of age, it can be assumed that each physician has ca. 1100–1500 people with lipid disorders under his/her care, including up to 10 patients with familial hypercholesterolaemia.
The prevalence of lipid disorders in Poland is still very high as compared to Western European countries, which, considering it is an independent cardiovascular risk factor, poses an immense challenge for the entire healthcare system.
5. Lipid disorders as a cardiovascular risk factor
Some lipoproteins present in the blood (i.e., LDL, lipoprotein (a) (Lp(a)), very low-density lipoprotein (VLDL) remnants and chylomicron remnants) are involved in all stages of atherogenesis, contributing to development of atherosclerotic cardiovascular disease (ASCVD) [35]. Therefore, lipid disorders in the form of increased plasma/serum concentration of analytes reflecting or associated with elevated atherogenic lipoprotein concentration are long-time recognised cardiovascular risk factors, based on the results of a huge number of experimental, epidemiological, and clinical studies [36].
A key role in the development of ASCVD is attributed to the disorders of low-density lipoprotein metabolism, and LDL-C concentration remains the primary test for detection and diagnosis of this group of lipid disorders (hypercholesterolaemia) and monitoring of lipid-lowering therapy [37]. The diagnostic role of non-HDL cholesterol and apolipoprotein B (apoB) concentration is similar, although it should be emphasised that non-HDL-C concentration, reflecting the blood level of all atherogenic lipoproteins, is a better predictor of cardiovascular risk than LDL-C concentration [38]. In certain situations, usually associated with metabolic disorders (Section 6), it is recommended to calculate non-HDL-C concentration or to determine the apoB concentration, alternatively or supplementary to LDL-C.
No reference intervals are established for plasma/serum LDL-C, non-HDL-C, or apoB concentrations. The interpretation of those results is based on their assumed target (desired) values, dependent on the total cardiovascular risk (Table V). For LDL-C, these values are lipid-lowering treatment goals.
Table V
Recommended categories of the total cardiovascular risk, modified and completed according to ESC/EAS 2019 recommendations [9] and PSDL/Pola 2020 guidelines [50]. The risk level indicates the presence of at least one of the factors listed in each category
| Extreme | Patient in primary prevention with Pol-SCORE > 20%1,2; status post-acute coronary syndrome (ACS) with another vascular incident within the past 2 years; status post-ACS and peripheral vascular disease or polyvascular disease3 (multilevel atherosclerosis); status post-ACS and concomitant multivessel coronary artery disease; status post-ACS and familial hypercholesterolaemia; status post-ACS in a patient with diabetes mellitus and at least one additional risk factor (elevated Lp(a) > 50 mg/dl or hsCRP > 3 mg/lor chronic kidney disease (eGFR < 60 ml/min/1.73 m2)) |
| Very high | Documented cardiovascular disease either clinical or unequivocal on imaging; type 2 diabetes mellitus with target organ damage4 or other major risk factors5,6, early-onset type 1 diabetes mellitus lasting > 20 years; chronic kidney disease with eGFR < 30 ml/min/1.73 m2; familial hypercholesterolaemia with cardiovascular disease or another major risk factor5; Pol-SCORE risk ≥ 10% and ≤ 20% |
| High | A significantly elevated single risk factor, especially TC > 8 mmol/l (> 310 mg/dl), LDL-C > 4.9 mmol/l (> 190 mg/dl), or arterial blood pressure ≥ 180/110 mm Hg; familial hypercholesterolaemia without other risk factors; diabetes without organ damage (regardless of duration)7; chronic kidney disease with eGFR 30–59 ml/min/1.73 m2; Pol-SCORE risk ≥ 5% and < 10% |
| Moderate | Pol-SCORE risk < 5% |
| Low | Pol-SCORE risk < 1% |
1 E.g. a 65-year-old woman, smoker, with systolic blood pressure 180 mm Hg and total cholesterol concentration 6 mmol/l (230 mg/dl) or a 60-year-old man with systolic blood pressure 160 mm Hg and total cholesterol concentration 7 mmol/l (270 mg/dl; estimated LDL-C > 190 mg/dl);
2 The same risk is recommended based on the SCORE2 or SCORE-OP based on the recent 2021 ESC Prevention Guidelines;
3 polyvascular disease (= multilevel atherosclerosis) – the presence of significant atherosclerotic lesions in at least two of three vascular beds, i.e. coronary vessels, cerebral arteries, and/or peripheral arteries;
The results of numerous epidemiological studies have demonstrated an inversely proportional relationship between plasma/serum high density lipoprotein cholesterol (HDL-C) concentration and the incidence of cardiovascular events; therefore, HDL were considered anti-atherogenic lipoproteins, and low HDL-C concentration was considered a cardiovascular risk factor [39, 40]. Studies on mechanisms of anti-atherogenic activity of HDL, mainly reverse cholesterol transport, have also led to the discovery of dysfunctional HDL, developed in inflammatory and/or oxidative stress conditions (as well as a result of glycation and other processes), with limited or nullified anti-atherosclerotic activity, or even with pro-atherosclerotic properties [41]. Furthermore, the results of studies on agents markedly increasing plasma/serum HDL-C concentration failed to demonstrate their beneficial effect on cardiovascular risk [42]. These observations dispelled the myth of “good cholesterol”, and the HDL-C concentration is not used in the assessment of cardiovascular risk, or as a goal of treatment of dyslipidaemia.
Triglycerides (TG) concentration is a significant cardiovascular risk factor. Even in moderate hypertriglyceridaemia (> 1.7 mmol/l (150 mg/dl)), being a chronic condition in many individuals with obesity, metabolic syndrome, or diabetes mellitus, intravascular remodelling of LDL particles with formation of small dense LDL (sdLDL) occurs, which may not be reflected by plasma/serum LDL-C concentration. SdLDL particles, readily oxidised and/or glycated, have potent atherogenic activity. Hypertriglyceridaemia accompanied by increased sdLDL fraction and decreased HDL-C plasma/serum concentration is referred to as atherogenic dyslipidaemia [43, 44]. Since blood sdLDL concentration is not routinely determined, hypertriglyceridaemia remains its main indicator.
Lipoprotein (a) is a recognised independent cardiovascular risk factor, mainly of ischaemic heart disease/myocardial infarction and aortic valve stenosis [45]. Lp(a) has interindividual structural variability, and isoforms occurring in specific individuals are genetically determined and have an indirect effect on plasma/serum concentration of this lipoprotein (Section 6.8). Its elevated concentration associated with a high cardiovascular risk occurs in up to 20% of the population, up to 30–40% of patients with atherosclerotic cardiovascular disease, and 30–40% of individuals with familial hypercholesterolaemia. Elevated values are also observed in pregnant women, which may affect prognosis associated with the risk of preeclampsia, pre-term labour, or low birth weight [45–47].
Atherosclerosis is a polyaetiological condition and, similarly to cardiovascular diseases being its result (ASCVD), depends on many risk factors. In addition to “classical” risk factors of atherosclerosis, known since the time of the Framingham Heart Study (FHS), i.e., dyslipidaemia, tobacco smoking, and arterial hypertension, these include obesity, prediabetes and diabetes mellitus, chronic kidney disease, persistent inflammation, sedentary lifestyle, and many others. According to the principle of primary and secondary prevention of cardiovascular events, i.e., detect and eliminate or control all possible risk factors, these should be identified, and the patient should be classified in the appropriate total cardiovascular risk category (Table V). The overall risk determines the management to control its factors, and in dyslipidaemia sets the goals of treatment (Section 7).
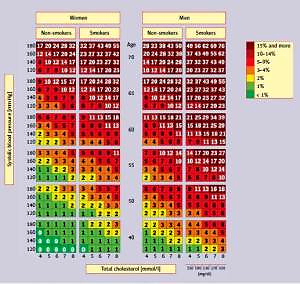
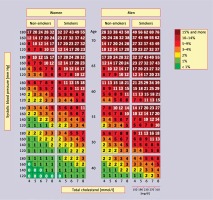
A cardiovascular risk assessment tool widely used in primary prevention, especially in the primary care setting, is the Pol-SCORE scale (Figure 2) [48], a modification of the SCORE (systemic coronary risk evaluation) scale developed by the European Society of Cardiology (ESC) experts. It is used to estimate the 10-year risk of cardiovascular death based on the patient’s sex, age, systolic blood pressure, smoking status, and plasma/serum total cholesterol concentration. The scale has been developed for people over 40 years of age and should not be used in patients with diabetes and/or chronic kidney disease.
Figure 2
SCORE tables calibrated for the Polish population (Pol-SCORE 2015) [48]. Numbers in the table represent 10-year risk of cardiovascular death
Evaluation of the total cardiovascular risk beyond the SCORE scale (Table V) requires extended diagnostics, including detailed clinical assessment, especially of the cardiovascular system, as well as tests concerning the carbohydrate metabolism/diabetes complications, renal function, etc. In the latest ESC/EAS 2019 guidelines [9] on the management of lipid disorders, the concept of extreme risk has been introduced to differentiate the risk among very high-risk patients (being a very heterogeneous group). Based on the results of available studies [48, 49], the definition of extreme risk was then extended in the PSDL/PoLA 2020 guidelines [50], and the current guidelines provide the optimum definition according to evidence-based medicine (EBM). Although achievement of therapeutic goals for this group (< 40 mg/dl/1.0 mmol/l) seems very difficult, introduction of this risk category draws attention to the need for intensive lipid-lowering therapy with immediate introduction of combination therapy (Section 9.8) to achieve as low LDL-C concentrations as possible (the lower the better), as soon as possible (the earlier the better). These recommendations also complement and definitely simplify the risk assessment in patients with type 2 diabetes mellitus.
In Table VI conditions associated with a higher cardiovascular risk not included in the Pol-SCORE 2015 table are presented. They should be considered, particularly if the cardiovascular risk assessed using the table is at the border between two categories (e.g., 5%). In this case, depending on HDL-C concentration or depression, a patient can be assigned to a higher (e.g., in the presence of depression or low HDL-C) or lower (e.g., in the absence of depression or high HDL-C) risk category. The Pol-SCORE tables should be a useful tool for patient education. They make it possible to easily present to the patient to what extent their cardiovascular risk will be reduced after introduction of effective treatment of a specific risk factor [8] (Table VII).
Table VI
Table VII
Recommendations concerning assessment of cardiovascular risk in patients with lipid disorders
| Recommendations | Class | Level |
|---|---|---|
| In each patient, overall cardiovascular risk should be assessed in order to adequately educate the patient and to make a decision on the need to initiate pharmacological treatment of dyslipidaemia and its intensity, including the need for the combination therapy. | I | A |
| The Pol-SCORE 20151, in which the 10-year risk of cardiovascular death is assessed, should be used to evaluate the overall cardiovascular risk in individuals in primary prevention. | I | A |
1 Risk analysis using the Pol-SCORE algorithm and tables is intended for primary prevention in individuals ≥ 40 years of age, without a history of cardiovascular events, and cannot be used to assess cardiovascular risk e.g., in people with type 2 diabetes or chronic kidney disease (GFR < 60 ml/min/1.73 m2), with direct assignment of such patients to the respective risk categories.
KEY POINTS TO REMEMBER
There is a close, independent relationship between cholesterol concentration, mainly non-HDL cholesterol and LDL-C, and development of atherosclerosis and risk of major cardiovascular events. In risk assessment, all cardiovascular risk factors should always be taken into account; when lipid goals have been achieved, these comprise so-called cardiovascular residual risk.
6. Recommendations on lipid profile measurement, its diagnostic significance, and limitations
The lipid profile performed to assess cardiovascular risk consists of assays/calculations of plasma/serum concentration of total cholesterol (TC), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), triglycerides (TG), and non-HDL cholesterol (non-HDL-C), and, as indicated, apolipoprotein B (apoB) and lipoprotein (a) (Lp(a)) [8, 35, 51, 52]. The results of these assays (except for Lp(a)) indirectly and approximately reflect the amount of respective lipoproteins in the blood. Of particular importance in laboratory assessment of lipid disorders and the risk of atherosclerosis progression is determination of blood content of atherogenic lipoproteins, i.e., LDL and Lp(a), although the latter is still very rarely determined [35]. Determination of chylomicron remnants (CM) and very low-density lipoprotein (VLDL) remnants with atherogenic activity is not yet used in clinical practice.
6.1. Pre-analytical issues
Tests constituting a lipid profile are performed using venous blood plasma/serum. It is considered that lipid profile assessment should be performed in conditions of normal daily activity and diet of a specific patient. Since people are not fasting for about 16 h a day, blood samples for routine testing do not need to be drawn in fasting conditions [9, 53, 54]. According to the 2016 position of the EAS and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM), a slight postprandial increase in TG concentration (up to 0.3 mmol/l (26 mg/dl)) does not significantly affect the assessment of lipid profile as compared with the same test in fasting conditions [35]. Small differences in interpretation of the results concern TG concentration, while the results of the LDL-C calculation using the Friedewald formula are consistent. It is recommended to consider repetition of the lipid profile assessment in fasting conditions with non-fasting TG concentration > 5 mmol/l (440 mg/dl) [35, 55].
The determined lipid concentrations are characterised by intra-subject variability of 5–10% for TC and > 20% for TG. In addition to genetic predispositions, variability in TC and TG concentration results from physical activity, diet, including carbohydrate and alcohol content, and smoking. Changes in lipid profile occur during pregnancy, particularly in the third trimester, mainly as an increase in TG (up to 3 times), TC and Lp(a) concentration, to a lesser extent, LDL-C (usually up to 50%) and HDL-C [8]. Higher TC and TG concentrations are observed in winter [51, 53, 55]. TC and LDL-C concentration is reduced for several weeks after a cardiovascular event and in chronic inflammation, e.g., in rheumatic diseases (lipid paradox), as well as in the elderly, especially those over 75 years of age [4, 56, 57].
Cholesterol and triglycerides are components of large molecule lipoproteins; therefore, maintenance of a compression band > 3 min or standing up for > 30 min before blood sampling may increase their concentration by 10–12% due to increased blood density, which should be avoided. Serum TC, HDL-C, LDL-C, and TG concentrations are approximately 3% higher than in plasma. Plasma/serum samples can be stored at a temperature of ~+4°C up to 4 days, while longer storage requires freezing at a temperature of –70°C [35].
6.2. Triglycerides
Triglycerides or triacylglycerols (TG) are esters of glycerol (an alcohol) and 3 molecules of fatty acids. TG are largely used as an energy source for the body and constitute the main component of fat cells [58]. They are synthesised endogenously and constitute most of the fat mass of food (exogenous origin – as chylomicron triglycerides) [58, 59].
Hypertriglyceridaemia reflects increased concentration of TG-rich lipoproteins, including atherogenic molecules (VLDL, CM remnants and VLDL remnants) leading to cardiovascular diseases, chronic inflammation, and increased overall mortality [60]. Increased TG concentration coexisting with low HDL-C concentration and high levels of small dense LDL particles is called atherogenic dyslipidaemia. Therefore, TG concentration is essential in the assessment of residual risk, as a high TG concentration even with the target LDL-C concentration significantly and independently increases cardiovascular risk [61–63]. In addition, very high hypertriglyceridaemia is associated with an increased risk of acute pancreatitis.
Plasma/serum TG concentration is measured using enzymatic assays and automated analysers [64]. The acceptable total error of TG measurement, as recommended by the US National Cholesterol Education Program (NCEP), is ±15%, and according to the Centre for Quality Assessment in Laboratory Diagnostics (COBJwDL), ±10% [50].
6.3. Total cholesterol
Cholesterol is obtained from food (~30%) or synthesised de novo, mainly in the liver and intestines (~70%). The amount of synthesised cholesterol depends on its level in the cells. Cholesterol affects the activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase by inhibiting its gene expression. The enzyme, HMG-CoA reductase, catalyses the key reaction in this pathway and is the site of action for statins. As the only de novo synthesised steroid, cholesterol is a substrate for the synthesis of steroid hormones, bile acids, and cardiotonic steroids (CTS). Cholesterol also plays an important role as a component of biological membranes, i.e., cytoplasmic and cell organelle membranes. Ca. 70% of blood cholesterol is transported by LDL; therefore, total cholesterol concentration indirectly and approximately reflects the amount of cholesterol in these plasma lipoproteins [50].
In clinical practice, TC concentration is used to stratify cardiovascular risk using the SCORE scale and to assess the severity of hypercholesterolaemia (suspected familial hypercholesterolaemia) and as the basis for therapeutic decisions in the absence of LDL-C calculation/test results (very rarely at present) [9, 65, 66]. Furthermore, the TC concentration must be known in order to calculate the LDL-C and non-HDL-C concentration. In medical laboratory practice, serum/plasma TC concentration is measured using enzymatic assays and automated analysers [67]. The acceptable total error of TC measurement, as recommended by the NCEP, is ±9%, and according to the COBJwDL – ±8% [50].
6.4. High density lipoprotein cholesterol
High density lipoproteins (HDL) are a heterogeneous group consisting of essentially two lipoprotein fractions of different particle size and density. In physiological conditions, HDL inhibit development of atherosclerosis mainly by their participation in reverse cholesterol transport from tissues, including macrophages in arterial walls, to the liver [68]. In addition, HDL have anti-oxidative activity and inhibit LDL oxidation [69], restore vascular endothelial function, and demonstrate anti-inflammatory and anti-apoptotic effects [70]. Inflammation and oxidative stress as well as glycation lead to changes in particle composition and dysfunctional HDL formation, with the loss of their anti-oxidative and anti-inflammatory properties and limitation of their activity in reverse cholesterol transport [71]. As a result, pro-atherogenic activity is attributed to dysfunctional HDL [71–73]. Laboratory tests used routinely to determine the HDL-C concentration in the blood do not make it possible to differentiate fractions (subfractions/subpopulations) or to assess functionality of these lipoproteins and therefore their role in atherogenesis in the examined patient. Methods of assessment of both heterogeneity and functionality of HDL are not available for routine laboratory diagnostics [35, 74–76].
Although an inverse relationship between blood HDL-C concentration and the risk of cardiovascular events has been demonstrated repeatedly, studies concerning agents increasing its concentration (i.e., niacin or cholesterol ester transfer protein (CETP) inhibitors) have not yet demonstrated their beneficial effects in terms of cardiovascular risk reduction [77, 78]. At present, HDL-C concentration is not recommended as a target in treatment of dyslipidaemia, a predictor of cardiovascular risk, or in monitoring of lipid disorders. However, HDL-C may be considered as an additional parameter in cardiovascular risk stratification using the SCORE scale. Nevertheless, HDL-C concentration remains an important element of the lipid profile as it is used to calculate LDL-C and non-HDL-C concentration [50].
Although plasma/serum HDL-C concentration brings only indirect information on the HDL blood content, it is still the main parameter in assessment of the number of HDL particles. Direct methods of measurement of the number of HDL particles (HDL-P) and their individual fractions (nuclear magnetic resonance spectrometry, ion mobility analysis, electrophoretic techniques) are not available for routine laboratory diagnostics. In addition, they do not provide sufficient new data to recommend them [50].
In diagnostic laboratories, enzymatic direct (homogenous) methods and automated analysers are commonly used for determination of plasma/serum HDL-C concentration. In these methods, detergents dissolving HDL and adsorptively blocking the access of enzymes to cholesterol in VLDL and LDL particles are used as reagents [35]. They are standardised and lower accuracy of measurements may be due to the matrix effect (media), e.g., in dyslipidaemias. According to the NCEP recommendations, the acceptable total error for direct HDL-C measurement methods is ±13% for normolipaemic samples and –20% to +36% for dyslipidaemic samples. Inaccurate results are mostly observed at HDL-C concentrations < 40 mg/dl (0.8 mmol/l). According to the COBJwDL, the acceptable error is ±15% [50].
6.5. Low density lipoprotein cholesterol
Cholesterol and its esters account for 40–50% of weight of the LDL particles that transport them to tissues. Due to the key role of LDL in the process of atherogenesis, the concentration of cholesterol contained in LDL (LDL-C), indirectly reflecting the blood LDL content, reflects significant cardiovascular risk [35, 50]. Specific LDL-C concentration values are the goal of lipid-lowering therapy. In addition, as it is not necessary to obtain blood samples for lipid profile testing in fasting conditions, the availability of LDL-C calculation/testing is increased [8, 9, 35, 50].
Direct methods of measurement of the number of LDL particles (LDL-P) are also not routinely applied, and in Poland they are actually only of scientific significance. In diagnostic laboratories, LDL-C concentration is usually calculated, but sometimes (although more often in recent years) measured using direct methods. The Friedewald formula is widely used to calculate LDL-C, using the determined TC, HDL-C and TG concentrations, and the assumed TG to VLDL-C ratio [79].
or
This formula should not be used for TG concentrations > 4.5 mmol/l (400 mg/dl) – in such conditions, the TG/VLDL-C ratio is different from the assumed. The results of calculations using the Friedewald formula are also less accurate if intermediate density lipoproteins (IDL) are present in the plasma as well as in conditions in which the composition of lipoprotein particles is changed (obesity, type 2 diabetes mellitus, metabolic syndrome, kidney, and liver diseases). The Friedewald formula also tends to decrease the results at low LDL-C concentrations < 1.8 mmol/l (70 mg/dl) and TG concentrations > 1.7 mmol/l (150 mg/dl) [80]. The calculated LDL-C concentration is also affected by the sum of errors of measurements used in the formula.
A modification of the Friedewald formula is the Martin and Hopkins formula (2013) [79]:
where x is the TG/VLDL-C ratio based on the concentration of TG and non-HDL-C; these values are available in special tables or online calculators, e.g., www.ldlcalculator.com.
It has been demonstrated that this formula is more accurate than the Friedewald formula for calculation of LDL-C at low concentrations and TG concentration in the range of 2.0–4.5 mmol/l (175–400 mg/dl), also in non-fasting samples [80–82]. The use of the Martin and Hopkins formula is limited by the need to purchase the license. Recently, a new formula for LDL-C calculation has been proposed, which provides more accurate results than both the above. The formula is more complex but compatible with modern laboratory IT systems. The new formula may be used in patients with low LDL-C concentration and those with significant hypertriglyceridaemia, up to 8.8 mmol/l (800 mg/dl) [83]. However, assessment of its practical use in laboratories will require time and further studies.
LDL-C concentration may be measured by enzyme-based direct (homogenous) methods using reagents containing detergents, surfactants, and other blocking components, or dissolving individual lipoprotein fractions, making LDL-C selectively available to the enzymes. The measurements are performed using automated analysers. The acceptable total error of measurement/calculation of LDL-C concentration, as recommended by the NCEP, is ±12 [50].
Currently, due to the limitations of the LDL-C concentration calculation described above, it is also recommended to calculate the non-HDL-C concentration or measurement of apoB concentration as an alternative to LDL-C concentration, and not its direct measurement [9, 35].
The calculated/measured LDL-C concentration is the sum of LDL-C and Lp(a) cholesterol concentration, which may result in elevated LDL-C concentration. The LDL-C concentration calculated using the Friedewald formula may be corrected for Lp(a) cholesterol using the Dahlen’s modification based on the assumption that cholesterol accounts for 30% of weight of Lp(a) particles [50, 84]:
This adjustment can be made for LDL-C concentrations determined in any other way.
6.6. Non-HDL cholesterol
Non-HDL cholesterol (non-HDL-C) concentration reflects the plasma content of all apoB-containing lipoproteins: LDL, VLDL, IDL, CM, CM remnants, VLDL remnants, and Lp(a), involved in the initiation of atherogenesis, and development and destabilisation of atherosclerotic plaques [85, 86]. As an indicator of atherogenic lipoprotein concentration, non-HDL-C concentration is very important for the assessment of cardiovascular risk and should be a permanent component of the lipid profile. This is of particular diagnostic importance if the accuracy of the LDL-C concentration calculation is limited. According to numerous studies, non-HDL-C concentration is more predictive for cardiovascular risk than LDL-C concentration [87, 88].
Non-HDL-C concentration is calculated using the following formula:
The calculated non-HDL-C concentrations are based on TC concentration calculated or determined using standardised methods and HDL-C concentration also determined by standardised direct methods. Nevertheless, the result of non-HDL-C concentration calculation is affected by the sum of errors of both measurements.
6.7. Apolipoprotein B
Apolipoprotein B (apoB), a component of all lipoproteins except HDL, occurs in two isoforms: apoB 100 present in VLDL, IDL and LDL, and apoB 48 (a fragment of apoB 100) present in CM and their remnants [9, 89]. Each LDL particle contains one apoB 100 molecule; therefore, the concentration of this apolipoprotein is a measure of the content of LDL particles in plasma/serum and a cardiovascular risk factor equivalent to the LDL-C concentration [90]. Measurement of the apoB concentration should be, in addition to calculation of the non-HDL-C concentration, an alternative to LDL-C calculation when its accuracy is reduced [9, 50]. Unfortunately, in Poland, apoB is still very rarely measured, which is due to the ongoing debate on the actual added value of this parameter in relation to LDL-C and non-HDL-C, as well as to additional costs of the test.
In laboratory practice, plasma/serum apoB concentration is determined using standardised immunoturbidimetric or immunonephelometric methods and automated analysers. The antibodies used in these methods are directed against apoB 100, although apoB 48 may also be measured using some of them. Possible interference with apoB 48 is marginal, as in the analysed material apoB 100 molecules, almost entirely LDL components, constitute of > 90% of apoB. The limit of acceptable error for apoB concentration measurement recommended by the NCEP is ±6% [50].
6.8. Lipoprotein (a)
Lipoprotein (a) (Lp(a)) particles are a subpopulation of LDL of similar structure, containing one apoB 100 molecule combined with apolipoprotein (a) [apo(a)]. There is significant inter-individual variability as to molecular weight of Lp(a), depending on the number of repetitions of the kringle IV type 2 (KIV-2) domain, ranging from 3 to 40, genetically determined. This genetically determined Lp(a) particle size is inversely proportional to the rate of its synthesis, mainly in the liver, and its plasma/serum concentration [9, 91].
In Poland, the concentration of Lp(a) is measured definitely too rarely, and the knowledge about it is still very limited. Therefore, every effort should be made to change it as soon as possible. This is necessary due to a great scientific progress in this field. Today we know that Lp(a) is an independent cardiovascular risk factor and that up to > 30% of patients with familial hypercholesterolaemia and/or acute coronary syndrome may have an elevated Lp(a) concentration, often with the desired LDL-C concentration, and there are options for pharmacological reduction of Lp(a) concentration [45, 92–95]. Therefore, we recommend that plasma/serum Lp(a) concentration should be measured once in every adult individual’s life to detect patients with its elevated concentration in whom the cardiovascular risk is high. Specific indications for Lp(a) measurement are: premature onset of cardiovascular disease, the lack of expected effect of statin therapy, and the need for better risk stratification in moderate- to high-risk individuals [50].
In diagnostic laboratories, plasma/serum Lp(a) concentration is determined mainly by immunochemical methods, i.e., immunoturbidimetric or immunonephelometric, or various immuno-enzyme methods, including ELISA. These tests may be performed routinely and readily available. Although the methods are standardised, sufficient harmonisation of results has not been achieved; this is considered a consequence of the impact of apo(a) particle size variation on the results of Lp(a) immunochemical assays [84, 96–98]. Therefore, for repeated Lp(a) concentration measurements the same method should be applied.
6.9. Laboratory report of the lipid profile
The lipid profile includes a set of blood plasma/serum tests discussed above performed for the diagnosis and monitoring of treatment of dyslipidaemia and to obtain a general picture of cardiovascular risk:
total cholesterol concentration (TC),
HDL cholesterol concentration (HDL-C),
LDL cholesterol concentration (LDL-C),
non-HDL cholesterol concentration (non-HDL-C),
triglycerides concentration
and ordered as indicated:
In addition to the measured/calculated results, the laboratory lipid profile report (Table VIII) should include information on how the LDL-C concentration was determined, as well as the target (desired) and alarm concentrations of individual analytes [100]. If severe dyslipidaemia is suspected, it should also contain information on the need for an urgent medical consultation in case of LDL-C concentration indicating a possible diagnosis of heterozygous (> 5.0 mmol/l, 190 mg/dl) or homozygous (> 13.0 mmol/l, 500 mg/dl) familial hypercholesterolaemia (FH), Lp(a) concentration > 180 mg/dl (450 nmol/l) indicating a very high risk of cardiovascular events, or TG concentration > 10.0 mmol/l (880 mg/dl) indicating a high risk of acute pancreatitis or suspected familial chylomicronaemia syndrome (FCS) [99]. It is helpful for interpretation and authorisation of the results by laboratory technicians to provide information on the referral form whether the patient is overweight/obese and/or suffers from diabetes, and whether they receive lipid-lowering therapy (Table IX).
Table VIII
Lipid profile – recommended contents of the laboratory report
| Parameter | Result [mg/dl] [mmol/l] | Target values | Alarm values |
|---|---|---|---|
| Total cholesterol (TC) | Fasting and non-fasting: < 190 mg/dl (5.0 mmol/l) | > 290 mg/dl (7.5 mmol/l)1 – suspected heterozygous FH | |
| HDL cholesterol (HDL-C) | Fasting and non-fasting: > 40 mg/dl (1.0 mmol/l) for men and > 45 mg/dl (1.2 mmol/l) for women | ||
| Triglycerides (TG) | Fasting: < 150 mg/dl (1.7 mmol/l); non-fasting: < 175 mg/dl (2.0 mmol/l) | > 880 mg/dl (10.0 mmol/l) – suspected familial chylomicronaemia syndrome (FCS) | |
| LDL cholesterol (LDL-C)2 | Fasting and non-fasting; cardiovascular risk: extreme < 40 mg/dl (1 mmol/l); very high < 55 mg/dl (1.4 mmol/l); high < 70 mg/dl (1.8 mmol/l); moderate < 100 mg/dl (2.6 mmol/l); low < 115 mg/dl (3.0 mmol/l) | > 500 mg/dl (13 mmol/l) – suspected homozygous FH (> 300 mg/dl [8 mmol/l] in patients on treatment); > 190 mg/dl (5.0 mmol/l) – suspected heterozygous FH | |
| Non-HDL cholesterol (non-HDL-C) | Fasting and non-fasting; cardiovascular risk: extreme < 70 mg/dl (1.8 mmol/l); very high < 85 mg/dl (2.2 mmol/l); high < 100 mg/dl (2.6 mmol/l); moderate < 130 mg/dl (3.4 mmol/l) | ||
| Apolipoprotein B (apoB) | Fasting; cardiovascular risk: extreme < 55 mg/dl (0.55 g/l); very high < 65 mg/dl (0.65 g/l); high < 80 mg/dl (0.8 g/l); moderate < 100 mg/dl (1.0 g/l) | ||
| Lipoprotein (a) [Lp(a)] | Fasting and non-fasting: < 30 mg/dl (75 nmol/l) | 30–50 mg/dl (75–125 nmol/l) moderate risk; > 50 mg/dl (125 nmol/l) high risk; > 180 mg/dl (450 nmol/l) very high cardiovascular risk |
1 FH – familial hypercholesterolaemia; in relation to the Simon Broome (UK) and MEDPED (US) FH diagnosis criteria [100];
Table IX
Recommendations concerning the lipid profile measurement
KEY POINTS TO REMEMBER
The lipid profile includes measurement of serum/plasma concentrations of TG, TC, HDL-C, LDL-C, non-HDL-C, and, as indicated, apoB and Lp(a).
In is not necessary to obtain blood samples for lipid profile testing in fasting conditions; repetition of the tests in fasting conditions should be considered at TG concentration > 5 mmol/l (440 mg/dl) in non-fasting conditions.
A high TG concentration even with the target LDL-C concentration attainment significantly increases cardiovascular risk (residual cardiovascular risk).
HDL-C concentration is not a predictor of cardiovascular risk or a target of lipid-lowering therapy.
LDL-C concentration is a key lipid parameter determining cardiovascular risk and a target of lipid-lowering therapy.
LDL-C concentration may be calculated using the Friedewald formula or Martin and Hopkins formula with TG ≤ 4.5 mmol/l (400 mg/dl); at low LDL-C concentration < 1.8 mmol/l (70 mg/dl) and TG > 2.0 mmol/l (175 mg/dl), Martin and Hopkins formula is recommended.
In individuals with TG concentration > 4.5 mmol/l (400 mg/dl), obesity, diabetes mellitus, metabolic syndrome, or low TC and LDL-C concentration, calculation of non-HDL-C or measurement of apoB concentration is recommended.
It is recommended to calculate non-HDL-C concentration along with calculation/measurement of LDL-C. It is a very important parameter in evaluation of cardiovascular risk; more predictive than LDL-C concentration.
Plasma/serum Lp(a) concentration should be measured once in every adult individual’s life to detect patients with elevated concentration increasing cardiovascular risk, especially those with extremely high Lp(a) levels ≥ 180 mg/dl (≥ 430 nmol/l) and therefore with a very high lifetime risk of ASCVD, approximately equivalent to the risk associated with HeFH.
The laboratory lipid profile report should include the results of measurements as well as the target (desired) and alarm concentrations of the analytes, and information on the need for urgent medical consultation.
7. Therapy goals of lipid disorders – target values depending on the risk
The most important parameter of the lipid profile is LDL cholesterol. This is due to several facts, well-known for a long time. Firstly, epidemiological studies have demonstrated a close relationship between cholesterol concentration and the risk of cardiovascular events, mainly coronary events [8, 9]. Secondly, experimental studies indicate the central role of cholesterol in the pathogenesis of atherosclerosis and its complications [8, 9]. Thirdly, it has been demonstrated that cholesterol present in atherosclerotic plaques is derived from LDL particles [8, 9]. Fourthly, intensive pharmacological reduction of LDL-C concentration results in regression of atherosclerosis [101–103]. Fifthly, reduction of cholesterol concentration is associated with a proportional reduction of the risk of cardiovascular events [104, 105]. For these reasons, reduction of LDL-C concentration is the main (primary) target of lipid-lowering therapy. However, in recent years it has also been unequivocally demonstrated that not only effective reduction of cholesterol concentration according to the rule of “the lower the better” is important, but that achievement of the therapeutic goal for LDL-C as soon as possible, according to the rule of “the earlier the better”, and maintaining it as long as possible (= “the longer the better”), is also of critical importance [2, 6, 7, 106].
No LDL cholesterol concentration has been identified below which no further benefits of lipid-lowering therapy can be observed (even for < 20 mg/dl or 0.5 mmol/l) or the risk of adverse effects outweighs the benefit [6, 8, 9]. It is worth emphasising, as the problem is often a matter of concern, that there is no evidence suggesting any risk of adverse effects, including neurocognitive disorders or haemorrhagic stroke, even for extremely low LDL-C concentrations [107, 108]. Since relative benefits of reduction of LDL-C concentration are largely independent of baseline cholesterol concentration [9, 105], absolute benefits are proportional to the overall cardiovascular risk. Therefore, the target LDL-C concentration depends on baseline cardiovascular risk (Tables X and XI). In relation to this, the authors of these guidelines decided to extend the definition of extreme cardiovascular risk in order to highlight the fact that very high-risk patients constitute a very heterogeneous group and with additional risk factors (i.e., higher risk) greater benefits of lipid-lowering interventions may be expected [109]. That is why it is so important to follow the rule of “the lower the better” in these patients and achieve the treatment goal as soon as possible [109]. The first data concerning this problem appeared after the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) study with simvastatin and ezetimibe [110]; then the definition of extreme risk, still based on expert opinion, was introduced in the guidelines of the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) guidelines in 2017 [111], while hard clinical data were provided by the results of the FOURIER study for evolocumab and ODYSSEY OUTCOMES study for alirocumab, with a number of sub-analyses [112, 113]. In March 2019, we summarised these results and identified patient groups that obtain the greatest benefit from treatment with PCSK9 inhibitors – assuming that these benefits are greatest for NNT (the number of patients who need to undergo a specific intervention for a defined period to prevent 1 event) < 30 [49], which was eventually partially reflected in September 2019 in the ESC/EAS guidelines [9]. However, these guidelines were surprising as they limited this group to patients with ASCVD and another vascular event in the previous 2 years [9]. Therefore, as soon as in March 2020, in the PTDL/PTL guidelines [50] this definition was extended by 3 other groups, and in the current guidelines, based on a large amount of recent scientific data, two further groups have been added, including patients in primary prevention with Pol-SCORE > 20% (Tables V and X). However, it seems, particularly in the context of the latest analysis of the TERCET registry, in which we attempted to validate all available definitions and select those risk factors that significantly increase the risk of another myocardial infarction in a 12- to 36-month follow-up period, that this definition may still be changed [114].
Table X
Recommended LDL-C concentrations as lipid-lowering treatment goals
| Recommendations | Class | Level |
|---|---|---|
| In secondary prevention patients with a very high cardiovascular risk, it is recommended to reduce LDL-C concentration to < 1.4 mmol/l (< 55 mg/dl) and by ≥ 50% of the baseline value. | I | A |
| In primary prevention patients with a very high cardiovascular risk, with or without FH, it is recommended to reduce LDL-C concentration to < 1.4 mmol/l (< 55 mg/dl) and by ≥ 50% of the baseline value. | I | C |
| In primary prevention patients with Pol-SCORE > 20% OR after an acute coronary syndrome (ACS) and another vascular incident within the previous 2 years OR after an acute coronary syndrome with peripheral vascular disease or polyvascular disease OR after an acute coronary syndrome with multivessel coronary artery disease OR after an acute coronary syndrome with familial hypercholesterolaemia OR after an acute coronary syndrome with diabetes mellitus and at least one additional risk factor (elevated Lp(a) > 50 mg/dl or hsCRP > 3 mg/l or chronic kidney disease (eGFR < 60ml/min/1.73 m2)), LDL cholesterol concentration < 1.0 mmol/l (< 40 mg/dl) may be considered as the target value1. | IIb | B |
| In patients with a high cardiovascular risk, it is recommended to reduce LDL-C concentration to < 1.8 mmol/l (< 70 mg/dl) and by ≥ 50% of the baseline value. | I | A |
| In patients with a moderate cardiovascular risk, reduction of LDL-C concentration to < 2.5 mmol/l (< 100 mg/dl) should be considered. | IIa | A |
| In patients with a low cardiovascular risk, reduction of LDL-C concentration to < 3.0 mmol/l (< 115 mg/dl) may be considered. | IIb | A |
1 Detailed explanations are provided in the legend of Table V.
Table XI
Intervention strategies as a function of total cardiovascular risk and untreated LDL-C concentration
The concentration of non-HDL cholesterol (a measure of cholesterol concentration in atherogenic lipoproteins, i.e., LDL, VLDL, and so-called remnants) and apolipoprotein B may be secondary goals of therapy, especially in patients with high triglyceride concentration. In these guidelines, we recommend the calculation of non-HDL cholesterol every time the lipid profile is performed. Adjustment of lipid-lowering treatment intensity in order to achieve target concentrations of non-HDL cholesterol (and apolipoprotein B in selected patient groups) may be considered in patients with a moderate, high, or very high cardiovascular risk, after the target LDL-C concentration has been achieved. Without calculation of non-HDL-C, having achieved the therapeutic goal for LDL cholesterol, we may omit a significant residual risk in our patient. In general, the target non-HDL cholesterol concentration should be approximately 0.8 mmol/l (30 mg/dl) higher than its equivalent LDL-C target.
The results of observational studies indicate that low HDL cholesterol concentration is a risk factor for ischaemic heart disease. However, it has not been decided whether it is a causal factor [71, 77]. According to the study results published so far, no reduction of the risk of cardiovascular events associated with the use of agents increasing HDL-C concentration has been observed [77, 78]. Therefore, HDL cholesterol concentration is not a treatment target; however, based on data from epidemiological studies, HDL-C < 1.0 mmol/l (< 40 mg/dl) in men and < 1.2 mmol/l (< 45 mg/dl) in women may be considered markers of increased risk, particularly in terms of SCORE risk estimation.
Available results of prospective and genetic studies as well as meta-analyses of numerous studies indicate that a high triglyceride concentration is an independent risk factor for cardiovascular events, especially in patients with diabetes mellitus or metabolic syndrome [115]. Scientific evidence indicating benefits of reduction of triglyceride concentration, especially with pharmacological treatment, is significantly poorer than the body of evidence for the benefits of LDL-C lowering. Although the relationship between triglyceride concentration and cardiovascular risk is continuous and triglyceride concentration is not a therapeutic target, a concentration < 1.7 mmol/l (< 150 mg/dl) is considered an indicator of low cardiovascular risk (Table XI).
8. Non-pharmacological prevention and treatment of lipid disorders
One of the primary targets in cardiovascular risk reduction is effective therapy of dyslipidaemia. LDL-C concentration is the most important parameter in diagnosing, prediction, monitoring, and treatment of lipid disorders [35]. According to current guidelines, once the patient’s cardiovascular risk has been estimated and the complete lipid profile has been determined, the management strategy recommended for the appropriate patient group should be selected. Lifestyle modification is recommended for all patients, and lipid-lowering agents are recommended only in selected clinical situations. In patients already treated, it is always a combination therapy, and its indispensable component is non-pharmacological treatment associated with lifestyle modification. The opinions expressed in the ESC/EAS 2019 guidelines on treatment of dyslipidaemia concur on this problem [9]. A summary of the effect of non-pharmacological modifications on individual lipoprotein fractions is presented in Table XII.
Table XII
Summary of the effect of non-pharmacological interventions on specific lipid profile parameters
8.1. Effect on TC and LDL-C/non-HDL-C
The greatest effect on reduction of total cholesterol and low-density cholesterol has decreased consumption of saturated fatty acids (SFA) and trans-fatty acids [9, 116]. The use of phytosterol-containing functional food has comparable effects on TC and LDL-C. Conversion of 1% of the energy from SFA to energy from monounsaturated fatty acids (MUFA) may reduce plasma LDL concentration by 1.6 mg/dl, and conversion to energy from polyunsaturated fatty acids (PUFA) – by 2 mg/dl [117–119]. Conversion from SFA to carbohydrates corresponds to the lowest reduction in LDL-C concentration – by 1.2 mg/dl. When switching from SFA-rich foods to carbohydrate-rich foods, products containing high amounts of fibre should be chosen. Fibre actively reduces intestinal fat absorption, and the energy density of most fibre-rich plant products is low, which makes it easier to maintain caloric balance. Weight reduction, as well as physical activity, does not significantly reduce LDL-C concentration (LDL-C is reduced by an average of 8 mg/dl for each 10 kg lost, and with intensive exercise it is possible to reduce LDL-C by approximately 5–7%), while both overweight and low physical activity are independent risk factors for cardiovascular disease; furthermore, modification of these factors significantly affects the reduction of TG and increase of HDL-C concentration [117–119].
8.2. Effect on TG
Among interventions aimed at reduction of triglycerides, weight reduction, regular physical activity, minimum alcohol intake, and decreased consumption of monosaccharides play the most important role [120]. Reduction of body weight and increased physical activity improve tissue insulin sensitivity, which directly affects plasma TG concentration. Intake of calories contained in alcohol can make it difficult to achieve normal body weight. Alcohol consumed in excess (more than 10–30 g/day) significantly increases TG concentration [118, 119, 121]. Conversion from SFA to unsaturated fatty acids (especially PUFA) significantly improves insulin sensitivity. Unfortunately, their intake is rarely sufficient with diet based on natural products; in such cases, supplementation of n-3 PUFA should be considered, which in the last few years has started to play a key role in treatment of hypertriglyceridaemia [122, 123].
TG concentration is also closely associated with impaired carbohydrate metabolism. Excessive intake of monosaccharides, including fructose (> 10% of food energy), significantly translates into increased TG concentration [120]. Fructose intake equivalent to 15–20% of food energy increases plasma TG concentration by up to 30–40% [124]. The best results in terms of reduction of plasma TG concentration are achieved with food products with a low glycaemic index (e.g., raw fruit, vegetables, thick groats, oat bran, cottage cheese, fish). Glycaemic index makes it possible to identify foods with a rapid glucose absorption profile and differentiate them from products from which carbohydrates are slowly absorbed to plasma. Fibre contained in plant products decreases the glycaemic index of food products by means of glucose absorption followed by its gradual release during intestinal transit [125].
8.3. Effect on HDL-C
High density lipoproteins with normal functionality have anti-atherosclerotic properties. Anti-atherosclerotic activity of HDL is mainly related to their participation in reverse cholesterol transport, but also with their anti-inflammatory, anti-oxidative, anti-apoptotic, anticoagulation, cytoprotective, vasodilatory, or even antitumour activity [126, 127]. HDL-C concentration provides no information on HDL functionality. Unfortunately, pharmacological attempts to increase concentration of these lipoproteins have not produced satisfactory effects in terms of cardiovascular risk reduction; therefore, only methods of behavioural medicine remain at present at our disposal. Lifestyle modification leading to weight reduction contributes to an increase in HDL-C plasma concentration by 0.01 mmol/l (0.4 mg/dl) for every kg lost. Systematic exercise of moderate intensity ca. 300 min per week may increase HDL-C concentration by 0.15 mmol/l (6 mg/dl) [128]. Each 1000 kcal used translates into an increase of HDL-C concentration by ca. 3 mg/dl. Smoking cessation is also beneficial, provided that it does not result in body weight increase [129].
The most significant HDL-C increase may be observed as a result of trans-fats intake reduction; moreover, unsaturated trans fats increase LDL-C concentration. An increase in HDL-C is observed when SFA intake is increased. Unfortunately, this increase is associated with an increase in LDL-C, which in turn does not produce a beneficial effect in terms of cardiovascular risk reduction and cannot be recommended. It should be emphasised that conversion from fat to monosaccharides as a food energy source results in reduction of HDL-C concentration. However, this effect was not observed with conversion to complex carbohydrates and fibre-rich foods (with a low glycaemic index) [116].
Alcohol consumption is a dietary habit inducing an increase of HDL-C concentration. However, it should be remembered that this applies only to moderate alcohol consumption (up to 30 g/day in men and up to 20 g/day in women), and its abuse is a risk factor of numerous diseases. Due to the risk of dependence and alcohol-related harm, its consumption should not be recommended to patients; moreover, recent analyses of the Global Burden of Disease (GBD) expert group clearly indicate that any amount of alcohol is harmful, and such a recommendation should be given to patients [130].
8.4. Significance of nutraceuticals and modified foods
Functional foods/nutraceuticals have potentially significant functional effect that helps to achieve therapeutic goals in terms of concentration of TC and individual fractions. Interestingly, most natural products have pleiotropic effects (although it is an incorrect name), affecting not only the lipid profile but also glucose concentration, insulin resistance, vascular stiffness, blood pressure, inflammation, or oxidative stress [131]. Moreover, as natural products, they are very safe, provided that production quality is maintained, and no impurities or additional substances are present (e.g., citrinin in red rice). Therefore, for several years, apart from their focus on the properties of nutraceuticals, manufacturers and experts have also very seriously treated safety, its monitoring and reporting the occurrence of all adverse reactions (nutrivigilance) [132, 133].
Below we present only a few examples of nutraceuticals with documented lipid-lowering properties; see Table XIII for a complete list. The experts of these guidelines have adapted with minor modifications the recommendations of the International Lipid Expert Panel (ILEP) on the use of nutraceuticals in treatment of lipid disorders [134–136].
Table XIII
Recommendations for the use of nutraceuticals in treatment of lipid disorders (adapted International Lipid Expert Panel 2017 guidelines with modifications [134, 135])
| Name | Recommended dosage | Expected LDL-C reduction | Class of recommendation | Level of recommendation |
|---|---|---|---|---|
| Inhibitors of cholesterol absorption from the intestine | ||||
| Plant sterols and stanols | 400–3000 mg | –8% to –12% | IIa | A |
| Soluble fibre (beta-glucan, psyllium, glucomannan) | 5–15 g | –5% to –15% | IIa | A |
| Chitosan | 1–6 g | –5% | IIb | A |
| Probiotics | Depending on bacterial strain | –5% | IIb | B |
| Inhibitors of hepatic cholesterol synthesis | ||||
| Red yeast rice extract | < 3 mg* | –15% to –25% | I | A |
| Garlic | 5–6 g (extract) | –5% to –10% | IIa | A |
| Pantethine (vitamin B5 derivative) | 600–900 mg | –5% | IIa | A |
| Bergamot | 500–1000 mg (polyphenol fractions, BPF) | –15% to –40% | IIa | B |
| Polycosanol | 10–80 mg | –18% to –25% | IIb | B |
| Inducers of LDL-C excretion | ||||
| Berberine | 500–1500 mg | –15% to –20% | I | A |
| Green tea extract | 25–100 g | –5% | IIa | A |
| Soy and lupin proteins | 25–100 g | –3% to –10% | IIb | A |
| Other nutraceuticals of mixed properties | ||||
| Polyunsaturated omega-3 fatty acids** | 2–4 g | – | I | A |
| Gamma-oryzanol | 300 mg | –5% to –10% | IIb | B |
| Spirulina | 400–800 UI | –5% to –10% | IIa | B |
| Curcumin | 0.5–3 g | –5% to –10% | IIa | A |
| L-carnitine | 1–2 g | – | IIb | B |
| Artichoke | 1–3 g (leaf extract) | –5% to –15% | IIa | B |
| Vitamin E | 400–800 UI | Up to –5% | IIb | B |
| Anthocyanins | 100–450 mg | –5% to –10% | IIb | B |
8.4.1. Phytosterols and stanols
The main phytosterols are sitosterol, campesterol, and stigmasterol. They are present naturally in vegetable fats, vegetables, fresh fruit, whole grain products, and leguminous plants. At present, they are added to certain margarines and yoghurts. Daily intake of 2 g of phytosterols or stanols (synthesised from plant sterols; naturally present in fruit, nuts, grain, and vegetable oils) translates into a reduction of LDL-C and TC concentration by ca. 7–10% [137, 138].
8.4.2. MUFA and PUFA
In this group, n-3 acids deserve particular attention. Their consumption in the amount of about 2–4 g/day translates into a TG reduction by ca. 25–30%, as well as a significant reduction of inflammatory markers. However, such supplementation may translate into only a small reduction of LDL-C concentration (ca. 5%). The latest data indicate that the effect of MUFA treatment (as well as the use of omega-6 acids) is usually neutral and does not translate into significant clinical benefits; therefore, dietary fat content should not as much be reduced as modified, with focus on their quality and replacement of SFA with omega-3 PUFA [123, 139]. The use of n-3 PUFA is recommended in treatment of hypertriglyceridaemia in patients with very high TG concentration as an adjuvant treatment, and the latest data, especially those concerning highly purified eicosapentaenoic acid (EPA – icosapent ethyl), also indicate that this effect, associated with a significant reduction of TG concentration, translates into a significant reduction in cardiovascular events by 25%. Therefore, the most recent recommendations point to the role of omega-3 acids, particularly in patients diagnosed with ASCVD [140] (Section 9.9).
8.4.3. Red yeast rice
The mechanism of action of pigments contained in fermented rice is identical to that of statins. This results from the fact that monacolin K contained in it is a molecule identical to lovastatin [141]. However, it should be remembered that marketed products containing monacolin K may differ with respect to dose, which translates into potency. Following a difficult to understand (taking into account ample data available) decision of the European Food Safety Authority (EFSA) of August 2018, the discussion on safety of the applied doses of monacolin K present in red rice continues [141]. The analyses performed since then indicate very high safety of the use of red rice, even in patients with statin intolerance, and the incidence of adverse events in consumer analysis is estimated at even less than 0.04% [132, 133]. The most recent draft ESFA recommendation indicates that formulations containing up to 3 mg of monacolin K will be commercially available.
However, these products (as well as others with demonstrated lipid-lowering properties) may provide a good solution for: (1) statin-ineligible patients receiving early pharmacotherapy of dyslipidaemia, (2) patients declining statin therapy, (3) statin-intolerant patients, and (4) all those who (both in primary and secondary prevention), despite available treatment, still fail to achieve the treatment goal.
8.5. Importance of healthy lifestyle
Overweight and obesity comprise one of the most common risk factors for dyslipidaemia in Polish population; in 2021, after more than a year of the pandemic, it may be present in up to 50% of Poles [144]. It is therefore postulated that achievement of normal body mass index (BMI), i.e., 18.5–24.9 kg/m2, should be pursued as one of the elements of struggle for normal lipoprotein concentrations. In addition to BMI, the patient’s waist circumference is important in defining obesity. Waist circumference (men ≥ 94 cm; women ≥ 80 cm), independent of normal BMI values, determines a higher risk of cardiovascular disease [8, 9].
Development of dietary habits should be based not only on correct balance of caloric supply and expenditure, but also on ensuring appropriate proportions of essential nutrients. Fatty acids should not exceed 30% of energy supply, and SFA should not exceed 10% of the total energy supply in individuals without dyslipidaemia; in patients with dyslipidaemia, dietary intake of SFA should be limited to a maximum of 7% (Table XIV) [142, 143, 147]. In regular diet, SFA should optimally be replaced with MUFA, and, above all, PUFA. This may be done with plant-based diets. Such development of dietary habits is consistent with the latest nutrition pyramid proposed by the World Health Organization (WHO). Development of dietary habits based on plant products also allows for a large dietary supply of fibre and reduced consumption of monosaccharides. Complex carbohydrates should account for up to 55% of total energy supply. Within carbohydrates, complex carbohydrates should predominate, while monosaccharides and disaccharides should not exceed 10% of energy supply [145–147] (Table XIV).
Table XIV
Summary of recommendations as to the optimal lipid-lowering diet for a patient with hypercholesterolaemia vs. high TG concentration
| Variable | Patient with hypercholesterolaemia | Patient with a high TG concentration |
|---|---|---|
| Fat intake | ||
| Carbohydrate intake |
| |
| Alcohol | ||
| Dietary supplements and functional food |
| |
| Dietary fibre |
| |
| Omega-3 unsaturated fatty acids |
|
|
| Preferred products in diet |
| |
Lifestyle medicine is one of the most important elements of prevention and treatment of lipid disorders. Modification of dietary habits, physical activity, avoiding of stimulants, and functional food supplementation play the most important role in this aspect. In view of current guidelines, lifestyle modification should be promoted in each patient group as a constant and inherent element of treatment of lipid disorders (Table XV) [148].
Table XV
Recommendations on healthy lifestyle as an essential component of treatment of lipid disorders
| Recommendation | Class | Level |
|---|---|---|
| Non-pharmacological modifications (so called lifestyle changes) are recommended in all patients with dyslipidaemia as an essential component of prevention and treatment. | I | A |
| Consumption of saturated fatty acids should be reduced to < 10% of energy supply, and in patients with hypercholesterolaemia to < 7% (the lower the better!). | I | B |
| Unsaturated trans fatty acids of natural origin should account for < 1% of caloric intake. Unsaturated trans fatty acids of industrial origin should be excluded. | I | C |
| At least 200 g of fruit should be consumed daily. | I | C |
| At least 200 g of vegetables should be consumed daily. | I | C |
| Fish should be consumed at least once or twice per week. | I | C |
| Regular exercise (preferably no less than 30 min every day) of moderate intensity, from 3.5 to 7 h per week, is recommended. Every increase in exercise intensity (regardless of baseline parameters) reduces the risk of all-cause mortality and death due to ischaemic heart disease [148]. | I | C |
9. Principles of pharmacological treatment of lipid disorders
9.1. Statins
Statins have been present in clinical practice for 30 years [149]. Currently, the two most potent agents in this group are preferred, i.e., atorvastatin and rosuvastatin. Pitavastatin, which in this year (2021) will appear on the Polish market, may also be used, especially as it is the 3rd most potent statin (in a dose of 4 mg it may reduce LDL-C concentration by up to 47%), with unique properties associated with reduction of triglyceride concentration, and with the best safety profile (although relatively poorly studied) [150]. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, a key enzyme in cholesterol synthesis in hepatocytes, resulting in increased LDL receptor activity on these cells and increased clearance of lipoproteins containing apolipoprotein B-100, i.e., LDL and VLDL remnants (including intermediate density lipoprotein – IDL) [35, 50]. In comparative studies, atorvastatin and rosuvastatin in a dose of 10 mg/d after 6 weeks of treatment reduced LDL-C concentration by 36.8% and 45.8%, in a dose of 20 mg/day by 42% and 52.4%, and in a dose of 40 mg/day by 47.8% and 55%, respectively [151]. Statins moderately reduce triglyceride concentration. Atorvastatin in a dose of 10 mg/day, 20 mg/day, and 40 mg/day by 20%, 22.6%, and 26.8%, respectively, and rosuvastatin at the same doses by 19.8%, 23.7%, and 26.1%, respectively. These two statins are most applicable in terms of achievement of the treatment goal for LDL-C, provided they are used regularly in appropriate doses [152]. Unfortunately, recent data have demonstrated that the highest dose of rosuvastatin (40 mg) is used in little more than 5% of patients, and that of atorvastatin (80 mg) in only 1.3%! (Figure 3).
Figure 3
Frequency of prescription of specific doses of atorvastatin and rosuvastatin in Poland (2017April 2021; data based on the IMS)
The importance of statins is different in specific patient groups, e.g., the safe statin in patients with chronic kidney disease is atorvastatin, while moderate doses of rosuvastatin are preferred in the elderly, due to the best safety profile of this agent in this group [153]. It should also be emphasised that currently we do not recommend the use of simvastatin (particularly in a dose of 80 mg due to adverse reactions), but if the treatment goal has been achieved and the patient tolerates it well, there is no need to change it.
Achievement of treatment goals for LDL-C depends on its baseline concentration, statin strength, and dose. Therefore, knowledge of the LDL-C reduction levels for specific doses and formulations is so important. This makes it possible to adequately plan the treatment (in the context of the possibility of achievement of the therapeutic goal) and initiate lipid-lowering treatment with adequate reduction potential, according not only to the rule of “the lower the better”, but also “the earlier the better”. For example, in an ACS patient with baseline LDL-C concentration of 127 mg/dl, it is known that statins may reduce LDL-C by an average of 50% and the treatment goal for this patient is < 55 mg/dl (1.4 mmol/l); therefore, statin monotherapy would be insufficient, so we should initiate treatment with a combination of statin with ezetimibe, which has the potential to lower LDL-C by an average of 65% (and for rosuvastatin 40 mg with ezetimibe 10 mg, even more than 70%). The guidelines concerning treatment of lipid disorders have been changed based on these assumptions in order to provide physicians with tools to achieve new therapeutic goals for patients with very high and extreme cardiovascular risk [109, 140].
By doubling the statin dose, an additional reduction of LDL-C concentration of only 6% is achieved. This relatively small additional lipid-lowering effect is supposed to be due to an increase in the activity of subtilisin/kexin type 9 proprotein convertase (PCSK 9) during statin therapy (a rebound mechanism associated with reduced LDL-C concentration and an increased number of LDL receptors on hepatocyte surface). This enzyme breaks down LDL receptors in hepatocyte lysosomes and, therefore, once LDL-C particles enter the liver cells, the receptors do not return to the cell membrane to bind to subsequent lipoproteins [154]. Furthermore, particularly in high-risk patients, combination therapy may be more effective than doubling the statin dose both in terms of LDL-C reduction (by more than 15 mg/dl) and the number of patients achieving treatment goals (almost 2.5 times more) [155].
The main adverse effects of statins are myotoxicity, temporary elevation of alanine aminotransferase activity, and new cases of diabetes mellitus (Section 10.13 and Section 11) [153, 156]. Muscle symptoms are the most common reason for treatment discontinuation. These include mild to severe pain, muscle stiffness, tenderness, cramps, and weakness. They may be accompanied (or not) by elevated creatine kinase (CK) activity. Factors predisposing to myopathy include, but are not limited to, age over 75 years, female sex, renal or hepatic impairment, hypothyroidism, alcohol abuse, excessive physical exercise, genetic susceptibility, perioperative period, and concomitant use of agents that inhibit statin metabolism, e.g. clarithromycin, erythromycin, azole antifungals, dilitiazem, verapamil, amiodarone, fibrates, cyclosporine, clopidogrel, or sulphonamides [8, 9, 153]; concomitant use of antiviral or anti-rheumatic agents should also be considered with care, especially in the current era of a pandemic, as they may also lead to intolerance [157]. Statins may have diabetogenic properties (especially in subjects receiving intensive statin therapy), but despite development of carbohydrate disorders or diabetes mellitus, statin therapy should not be discontinued due to its net benefit (up to 5 times) in terms of cardiovascular risk reduction [158]. The management should include glucose-lowering diet, weight reduction, if this is excessive, and possible use of antidiabetic agents [8, 9, 153].
In multiple randomised clinical trials, strong evidence for high efficacy of statins in primary and secondary prevention of cardiovascular disease has been provided. The lower LDL-C concentration was achieved, the lower was the risk of cardiovascular events. This relationship was clearly confirmed by a meta-analysis of 26 randomised clinical trials concerning statins [159]. In 21 studies (a statin vs. another intervention, mainly placebo) involving 126,526 patients, a reduction of LDL-C concentration by 1 mmol/l (~40 mg/dl) was found to significantly reduce cardiovascular events by 21%. In the same study it was shown that intensive statin treatment, as compared to moderate (5 studies involving 39,612 patients), provided a significant 28% reduction in cardiovascular events per 1 mmol/l of LDL-C reduction (~40 mg/dl). Statin therapy reduces the incidence of stroke, coronary mortality, and overall mortality [159].
KEY POINTS TO REMEMBER
Statins are the gold standard - first-line agents in treatment of hypercholesterolaemia, and among lipid-lowering agents have the best documented efficacy in primary and secondary prevention of cardiovascular diseases.
Due to the need to achieve the LDL-C treatment goal, atorvastatin and rosuvastatin are currently the most applicable agents. Unfortunately, due to the therapeutic inertia and lack of adherence, the doses in which they are most frequently used by patients are too low, what results in the lack of achievement of the therapeutic goal in 2/3 of patients [30].
During treatment, one should pay attention to adverse effects reported by the patient, mainly muscle symptoms, and if these occur, follow the recommendations, and try not to discontinue statin therapy.
9.2. Ezetimibe
Ezetimibe selectively inhibits the absorption of cholesterol from the small intestinal lumen to enterocytes by selective blocking of the NPC1L1 (Niemann-Pick C1 like 1) sterol transport protein [160]. As a result of reduced absorption, less cholesterol reaches the liver and consequently the expression of LDL receptors (LDLR) on the surface of hepatocytes is increased, thus increasing liver uptake of endogenous cholesterol contained in LDL lipoproteins [160]. Ezetimibe monotherapy in a dose of 10 mg reduces LDL-C concentration by 15–25%; however, quite a high inter-individual variability is observed [161]. This is determined by dietary variability (the lipid-lowering effect of the agent is increased with a high-cholesterol diet) and probably the variability of genes encoding NPC1L1; therefore, the response to ezetimibe alone may be significantly better in a certain group of patients [162]. This agent reduces TG concentration by 1.7–9.4% and increases HDL-C concentration to a small extent by 1.3–6.2% [163]. However, data on the effect of ezetimibe on lipoprotein (a) are inconsistent, although all indicate a numerical Lp(a) reduction (from 2.6 to 7.1%) [164, 165]. Nevertheless, following a meta-analysis by Tsimikas et al. [166] indicating a moderate but statistically significant (although probably clinically insignificant) increase of Lp(a) concentration following statin treatment by 6–7%, particularly in high-risk patients with elevated concentration of this lipoprotein, combination therapy with a statin and ezetimibe is recommended [167].
Combination treatment with ezetimibe and a statin, as a result of a synergistic effect, results in greater LDL-C concentration decrease than monotherapy with either agent [168]. Ezetimibe added to a statin reduces LDL-C concentration by another 15–20%; therefore, a combination of high-intensity statin treatment (i.e., atorvastatin or rosuvastatin at their highest doses) with ezetimibe can reduce LDL-C concentration by up to 65–70% [8, 9]. This combination is more effective (by more than 15 mg/dl) in terms of LDL-C reduction and 2.45 times more effective in achieving the treatment goal as compared to doubling the statin dose [155, 168]. Unfortunately, the combination of a statin with ezetimibe is still very rarely used not only in Poland and in Europe, but also worldwide, even though for 4 years ezetimibe has been a generic and very cheap product. In the Da Vinci study, the combination therapy was used only in 9.2% of patients [30], whereas in Central and Eastern European countries, in 7% [31]. This is only a small increase from the 2016/2017 data in which, based on the TERCET registry, combination therapy with a statin and ezetimibe was used only in less than 3% of ACS patients [169] (Figure 4).
Figure 4
Prescription of rosuvastatin in combination with ezetimibe (fixed combination) in Poland between May 2019 and April 2021 (based on the IMS)
In published randomised trials with ezetimibe, high lipid-lowering efficacy and favorable safety profile of combination therapy in patients with familial hypercholesterolaemia, renal failure, type 2 diabetes mellitus, metabolic syndrome, high cardiovascular risk, and ACS was demonstrated [8, 9, 170, 171]. In all these studies, in the group receiving combination therapy, the target LDL-C concentration was achieved significantly more often, and greater reduction of TC, non-HDL-C, TG and ApoB concentration was observed than with statin monotherapy [8, 9]. In addition, the results of IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) study demonstrated that LDL-C reduction with ezetimibe significantly reduces the incidence of cardiovascular events, and the higher the patient’s baseline cardiovascular risk, the higher the reduction [170, 171].
Ezetimibe is rapidly absorbed from the gastrointestinal tract, mainly as the pharmacologically active ezetimibe glucuronate. Simultaneous food intake does not significantly affect its bioavailability. It is metabolised in the intestine and the liver. It is neither an inducer nor an inhibitor of cytochrome P450 [8, 9]. Therefore, it does not interact with drugs metabolised by this cytochrome. Ezetimibe concentration profile has several daily peaks; therefore, time of administration does not affect its activity [172]. The agent is mainly (78%) eliminated with faeces, to a lesser extent (11%) with urine. Pharmacokinetic parameters of ezetimibe in the elderly and in patients with renal or mild hepatic insufficiency are not significantly altered [172]. It is contraindicated in patients with severe hepatic impairment. In contrast to agents interrupting bile acid reabsorption, ezetimibe does not impair the absorption of fat-soluble vitamins, thyroid hormone, warfarin, or β1-adrenolytic drugs. Ezetimibe inhibits the absorption of plant stanols and sterols, so these agents should not be used concomitantly [8, 172]. Data discussed above indicate that ezetimibe is an agent with a high lipid-lowering efficacy and a very good safety profile (it may also be used in pregnant women if benefits outweigh the risk); therefore, it should be used commonly, especially in the context of a very low percentage of patients achieving the therapeutic goal for LDL cholesterol in Poland (17% of very high risk patients achieve concentrations below 55 mg/dl (1.4 mmol/l) and in every 4th patient regardless of the risk) [30, 31].
KEY POINTS TO REMEMBER
Combination therapy with a statin and ezetimibe (intensive lipid-lowering therapy) should be the gold standard of care for patients at very high and extreme risk (Section 9.8) as it significantly increases the chances of achieving new therapeutic LDL-C targets.
High intensive statin plus ezetimibe provides very significant reduction of LDL-C concentration (by a mean of 65%) with a preserved or even better safety profile than high-intensity statin monotherapy.
Ezetimibe monotherapy should be considered immediately in patients with complete statin intolerance (without waiting for statin rechallenge) (Section 11), and in case of partial intolerance, in combination with the highest statin dose tolerated by the patient.
9.3. PCSK9 inhibitors
PCSK9 is a protein responsible for the metabolism of LDL-C bound to LDL-C receptors present in the blood and on the surface of liver cells [173]. This protein binds to LDLR on the hepatocyte surface and, along with LDL-C, undergoes endocytosis and then degradation of these complexes in liver cell lysosomes. This leads to reduced expression of LDL receptors on hepatocyte surface and in the blood, and therefore increases LDL-C concentration [173]. The discovery of this mechanism was accidental and concerned patients with very high LDL-C concentration and diagnosed with autosomal dominant hypercholesterolaemia (a gain of function mutation of PCSK9) as well as those with very low LDL-C (< 20 mg/dl) with a loss of function mutation as a result of which non-functional PCSK9 did not bind to LDLRs, resulting in the receptors’ return to the hepatocyte surface and effective LDL-C reduction [174]. This was the starting point for studies on various ways of PCSK9 inhibition (using monoclonal antibodies or RNA interference) that could support statins in effective LDL-C reduction.
Studies with PCSK9 inhibitors (evolocumab and alirocumab) were conducted in 3 patient groups, i.e., those at high cardiovascular risk, patients with familial hypercholesterolaemia, and those with statin intolerance [173]. In these studies, high effectiveness of the analysed agents in reducing LDL-C concentration (from 45% to 65% depending on the patient group versus placebo and by ca. 35% to 45% compared with ezetimibe), allowing up to 80-90% of patients in these groups to achieve their treatment goals, has been confirmed. Furthermore, PCSK9 inhibitors are also effective with respect to other lipid profile parameters, effectively reducing non-HDL-C concentration (on average by ca. 50% vs. placebo), apoB (ca. 50%), TG (15–20%), and Lp(a) (ca. 25%), as well as increasing HDL-C (5–10%) and apoA1 (3–5%) [173, 175]. Available studies indicate that PCSK9 inhibitors used in monotherapy may reduce LDL-C by 60% an average and used in combination with statins and ezetimibe by up to 85% [8, 9].
These agents (alirocumab and evolocumab) have been approved by both the US FDA and the European Medicine Agency (EMA) in the following indications: for use in adults with primary hypercholesterolaemia (familial heterozygous and non-familial) or mixed dyslipidaemia in addition to diet: (1) in combination with a statin or a statin and other lipid-lowering agents in patients, in whom the target LDL-C concentration cannot be achieved with the highest tolerated dose of a statin, or (2) alone or in combination with other lipid-lowering agents in statin-intolerant patients or those in whom statins are contraindicated. As evolocumab has been studied in patients with homozygous familial hypercholesterolaemia (the TAUSSIG and TESLA studies), it should also be considered in combination with other lipid-lowering agents in adults and adolescents aged at least 12 years with homozygous FH [175].
Both the FOURIER study [176] with evolocumab and the ODYSSEY OUTCOMES study [177] with alirocumab confirmed high efficacy of both PCSK9 inhibitors in terms of reduction of the primary endpoint (by 15%), and for alirocumab they demonstrated that PCSK9 inhibitors can also significantly reduce all-cause mortality (also by 15%). Subsequent sub-analyses, in subgroups of patients with a history of myocardial infarction and stroke, or several cardiovascular events, or an epidemiologically recent MI, or MI and concomitant peripheral vascular disease or multibed disease, post-MI patients with other risk factors, such as diabetes mellitus or elevated concentration of hsCRP or Lp(a), those with different baseline LDL-C concentration, or, finally, in patients with a long follow-up period (> 3 years), not only confirmed their high efficacy [178], but also provided the basis for identification of patients with extreme cardiovascular risk and creation of a reimbursement programme which since November 1st, 2018, has been available for patients with familial hypercholesterolaemia, and since November 1st, 2020, for patients post myocardial infarction. Unfortunately, the adopted reimbursement criteria make it possible to include only about 5–7% of patients with FH (due to the required high LDL-C concentration despite treatment) and a relatively small group of post-MI patients (mainly due to the need to include them within 12 months of MI onset). Due to all the above, at the time of preparation of these guidelines approximately 200 patients in total, mostly those with FH (a little more than 150) in nearly 30 centres in Poland (the list is available on PoLA website: https://ptlipid.pl/2020/09/28/osrodki-w-osrodki-w-polsce-w-polsce-w-ktorych-jest-realizowany-program-lekowy-ktorych-jest-realizowany-program-lekowy-leczenie-hipercholesterolemii-rodzinnej-icd-10-e78-01/) have been included into the therapeutic programme. As a result of intensive activity of the Societies (PoLA, PSC), experts, and patient organisations, the criteria have been changed since September 1st 2021, currently enabling treatment of patients with FH as early as at LDL-C > 100 mg/dl (2.5 mmol/l) and after not 6 but 3 months of prior statin and ezetimibe therapy (Table XVI).
Table XVI
Therapeutic programme: treatment with PCSK9 inhibitors in patients with lipid disorders (ICD-10 E78.01, I21, I22, I25)
The results of studies confirming a high efficacy of PCSK9 inhibitors administered immediately after an ACS (the EVOPACS and EVACS studies with evolocumab [179, 180] and the VCU-alirocRT study with alirocumab [181]) are also worth noting, as they were the starting point for recommendation concerning initiation of treatment with PCSK9 inhibitors during hospitalisation (recommendation level IIa C) in the most recent ESC/EAS 2019 guidelines [9]. The EVACS study demonstrated that the use of evolocumab immediately after an ACS was associated with significant LDL-C reduction as early as after 3 days (mean concentration 1.3 mmol/l) and below 1 mmol/l (40 mg/dl) after 4–7 days, as compared with the control group. Such early treatment resulted in 65.4% of patients at discharge and more than 85% after 30 days achieving their LDL-C target concentration below 55 mg/dl [180].
Studies performed to date do not indicate any significant adverse effects of PCSK9 inhibitors compared to statins and/or ezetimibe. Injection site reactions (redness and soreness) may be observed occasionally. In addition, effects typical for monoclonal antibodies may be observed, including: nasopharyngitis (< 5%), upper respiratory tract infections (ca. 3%), back pain (ca. 3%), arthralgia (ca. 2%), flu-like symptoms (ca. 2%), and nausea (ca. 2%). Despite numerous studies and much discussion, no increased risk of muscle symptoms (myalgia and myopathy), elevated liver enzymes or creatine kinase, or the risk of new cases of diabetes mellitus or cognitive dysfunction has been confirmed [9, 49, 182]. With reference to the assessment of cognitive risk, the EBBINGHAUS study with evolocumab enrolled 1204 patients followed up for a mean of 19 months [182–184]. No differences between the groups (evolocumab vs. placebo) were observed, either with respect to the primary endpoint (Spatial Working Memory Index) or to the secondary endpoints, i.e., the results for working memory, episodic memory, and psychomotor speed. Exploratory analysis revealed no association between LDL-C concentration and cognitive changes [182–184].
KEY POINTS TO REMEMBER
Based on the results of the FOURIER and ODYSSEY OUTCOMES studies and their sub-analyses, PCSK9 inhibitors are recommended in secondary prevention in very high-risk patients who do not achieve their target with the maximum tolerated statin dose and ezetimibe.
PCSK9 inhibitors are also recommended in very high-risk patients with FH (i.e., those with ASCVD or another major risk factor) who do not achieve their target with the maximum tolerated statin dose and ezetimibe.
Available data also demonstrate the importance of PCSK9 inhibitors in primary prevention that may be considered in very high-risk patients (but without FH) if the LDL-C target has not been achieved with the maximum tolerated statin dose and ezetimibe.
PCSK9 inhibitors should be introduced as soon as possible (after 4–6 weeks if the treatment goal has not been achieved) in patients with extreme cardiovascular risk in whom treatment should be started with a combination of a statin and ezetimibe (Section 9.8).
Studies performed so far have not indicated any significant adverse effects of this class of agents.
9.4. Fibrates
The mechanism of action of fibrates depends on the activation of transcription factors called peroxisome proliferator-activated receptors-α (PPAR-α) [185]. Fibrates are ligands of PPAR-α and peroxisome proliferators. By activating PPAR-α, these agents affect the expression of key genes encoding proteins involved in lipid metabolism. Reduction of triglycerides concentration is associated with activation of oxidative enzymes which increase oxidation of fatty acids in the liver, resulting in decreased lipid synthesis, and with increased activity of lipoprotein lipase (LPL), an enzyme on the vascular endothelium responsible for hydrolysis of triglycerides, and thus their catabolism. Fibrates increase synthesis of apolipoproteins A-I and A-II, two proteins present in HDL cholesterol [8, 185].
Fibrates reduce TG concentration by 25–50% and increase HDL-C by 10–25%. In cases of severe hypertriglyceridaemia, treatment should be initiated with a fibrate in order to quickly reduce serum lipid concentration as it is a risk factor for acute pancreatitis, which increases the risk of serious complications, including death [8, 185]. In large clinical trials with fenofibrate, in patients with diabetes (FIELD, the Fenofibrate Intervention and Event Lowering in Diabetes, and ACCORD, Action to Control Cardiovascular Risk in Diabetes) randomised to receive the active agent no effect on cardiovascular risk was observed as compared to placebo [186, 187]. However, such benefits (i.e., reduction of cardiovascular events) were observed in subgroups of patients with atherogenic dyslipidaemia (increased TG and decreased HDL-C concentration) and those with micro- and macroangiopathic complications (retinopathy or diabetic nephropathy) [186, 187]. Furthermore, both studies were subject to significant methodological errors; the largest study concerning statins (i.e., the Heart Protection Study, HPS) which demonstrated significant benefits of statin therapy in patients with diabetes was published during the FIELD trial, resulting in twice as many patients receiving statins in the placebo arm than in the fenofibrate arm. After adjustment of the results, i.e., exclusion of patients treated with statins in both groups, fenofibrate significantly reduced the primary endpoint of the FIELD study [115, 186]. Similarly, the ACCORD study, in which patients virtually optimally treated with statins with slightly elevated TG concentration were enrolled, also eventually failed to demonstrate reduction of the primary endpoint (excluding patients with atherogenic dyslipidaemia) [115, 187]. In the ACCORDION study, being continuation of the ACCORD study, 4644 subjects were enrolled, including 35% of patients with prior cardiovascular events. Only 4.3% of the study participants continued treatment with fenofibrate after completion of the ACCORD study [188]. In a follow-up period of 9.7 years, the hazard ratio (HR) for the primary endpoint in patients originally randomised to fenofibrate as compared to placebo was 0.93 (95% CI: 0.83–1.05; p = 0.25), i.e., comparable to the value originally observed in the ACCORD study [187, 188]. Again, it was observed that in patients with atherogenic dyslipidaemia (TG > 204 mg/dl and HDL-C < 34 mg/dl), fenofibrate treatment significantly reduced the risk of cardiovascular complications by 27% (HR = 0.73; 95% CI: 0.56–0.95) [188]. Unfortunately, no large clinical trials with fibrates used exclusively in patients with atherogenic dyslipidaemia have been performed.
The only fibrate available in Poland is fenofibrate, which has the highest number of studies; in other countries bezafibrate is also used, which, similarly to fenofibrate, is highly efficacious in reduction of TG concentration, and its effect on reduction of cardiovascular incidents has been confirmed [189]. Considering a growing percentage of individuals with metabolic disorders in Poland, this agent should also be available on the market for treatment of hypertriglyceridaemia. It is also worth mentioning that research on a new fibrate, i.e., pemafibrate, is ongoing; this agent may prove the strongest in the entire group in terms of TG reduction. In the PROVIDE trial, the efficacy and safety of pemafibrate in subjects with type 2 diabetes and hypertriglyceridaemia was evaluated in a period of 52 weeks [190]. The patients were randomised to placebo or pemafibrate 0.2 or 0.4 mg/day for 24 weeks; the treatment was then continued in all patients through week 52. The percentage changes in fasting serum TG concentration at the end of the study were –48.2%, –42.3%, and –46.4% for placebo/pemafibrate 0.2 mg/day, and pemafibrate 0.4 mg/day, respectively. Pemafibrate was well tolerated throughout the study [190]. The ongoing PROMINENT study (Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN patiENts With diabeTes), in which 10,000 patients with diabetes mellitus, mild to moderate hypertriglyceridaemia (TG: 200–499 mg/dl), and low HDL-C (≤ 40 mg/dl) will be enrolled and treated with pemafibrate (0.2 mg BID) for a maximum of 5 years (mean: 3.75 years), may answer the question of how this effective TG reduction will translate into reduction of cardiovascular events [191].
The most recent ESC/EAS 2019 guidelines [9] on the management of dyslipidaemia allow consideration of combination therapy with a statin and fenofibrate if TG concentration during statin therapy is still above 200 mg/dl (> 2.3 mmol/l).
Adverse effects of fibrates are generally moderate and rarely observed. Myalgia and myopathy (in combination with high-dose statins) as well as increased aminotransferase activity have been reported. These agents increase creatinine concentration. It should be known that fibrates are in 60–90% excreted renally, which limits their use in chronic kidney disease. Increased homocysteine concentration, cases of acute pancreatitis, and thromboembolism were also observed [8, 115].
KEY POINTS TO REMEMBER
The main indication for the fibrate therapy is severe hypertriglyceridaemia. In this case, fibrates are the first-line agents.
In patients with hypertriglyceridaemia statins are the first-line agents. Addition of a fibrate to a statin should be considered in patients with persistent hypertriglyceridaemia (TG > 200 mg/dl or 2.3 mmol/l) despite statin therapy.
9.5. Omega-3 acids
The importance of omega-3 has been discussed in detail in Section 8.4. It should be emphasised that their role in treatment of hypertriglyceridaemia has changed significantly over the last few years, especially after the REDUCE-IT (the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) study was published; it concerned highly purified eicosapentaenoic acid (EPA) (icosapent ethyl) which in a dose of 4 g/day demonstrated high efficacy in both primary prevention (in patients with diabetes and other risk factors) and secondary prevention in patients with ASCVD, reducing the primary endpoint of the study by 25% (HR = 0.75; 95% CI: 0.68–0.83; p < 0.001) [147]. It was also observed that cardiovascular mortality was significantly lower in the icosapent ethyl group than in the placebo group (4.3% vs. 5.2%; HR = 0.80; 95% CI: 0.66–0.98; p = 0.03). As to safety, a higher proportion of patients in the icosapent ethyl group were hospitalised due to atrial fibrillation or flutter (3.1% vs. 2.1%, p = 0.004) [147]. Further studies demonstrated the effect of icosapent ethyl on reduction of atherosclerotic plaque volume. The EVAPORATE study (Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy) enrolled patients with coronary atherosclerosis (≥ 1 angiographic stenosis ≥ 20%) treated with statins with LDL-C concentration 40–115 mg/dl and persistent high triglyceride concentration (135–499 mg/dl). In a 9-month analysis, a significant effect of omega-3 acids on atherosclerotic plaque morphology (i.e. increased plaque calcification, as well as reduction of the fibrous part and total volume of the plaque) was demonstrated [192].
Interestingly, these results have not been confirmed in subsequent studies with the mixture of omega-3 acids (EPA and docosahexaenoic acid – DHA). The VITAL study included nearly 26,000 individuals (in primary prevention, aged > 50 years for men and > 55 years for women) who were treated with vitamin D3 (2000 IU daily) and n-3 fatty acids of marine origin (1 g/day). The use of omega-3 acids did not significantly affect the study endpoints; only significant reduction in the risk of myocardial infarction was observed (HR = 0.72; 95% CI: 0.59–0.90) [193]. As noted in the comments, negative results of the study could be associated with a low-risk patient population (primary prevention), the form of omega-3 acids used (mixture), or a low dose used in the study.
Therefore, in a subsequent STRENGTH (A Long-Term Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh Cardiovascular Risk PatienTs with Hypertriglyceridemia) study the effect of a preparation containing EPA and DHA carboxylic acids in a dose of 4 g/day was investigated in over 13,000 patients with high cardiovascular risk and atherogenic dyslipidaemia treated with statins. Interestingly, in the study corn oil was used as placebo, which might have had an impact on the results of the study. The primary composite endpoint comprised cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization. When 1384 patients experienced the primary endpoint (of the planned 1600 events), the study was prematurely terminated based on an interim analysis that demonstrated low probability of clinical benefit from the use of omega-3 CA vs. the comparator applied. The primary endpoint occurred in 785 (12.0%) omega-3-treated patients compared with 795 (12.2%) corn oil-treated patients (HR = 0.99; 95% CI: 0.90–1.09; p = 0.84) [194]. In the omega-3 group, a significant reduction in TG concentration by 19% and hsCRP by 20% in comparison with the control group was observed [194].
A meta-analysis summarising studies concerning omega-3 acids published in recent years, which finally included 13 studies covering 127,447 individuals, demonstrated significant reduction of the risk of death due to ischaemic heart disease (risk ratio (RR) = 0.91, 95% CI: 0.85–0.97, p = 0.010), major vascular events (RR = 0.95, 95% CI: 0.93–0.98, p = 0.001), nonfatal myocardial infarction (RR = 0.89, 95% CI: 0.83–0.95, p = 0.001) and all-cause mortality (RR = 0.95, 95% CI: 0.92–0.99, p = 0.025) [195].
The REDUCE-IT study significantly changed the view on omega-3 fatty acids and their use in treatment of hypertriglyceridaemia. In December 2019, the FDA approved an icosapent ethyl formulation (Vazkepa) for treatment of hypertriglyceridaemia in order to reduce cardiovascular risk in high-risk patients [196]. In January 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion recommending marketing authorisation of Vazkepa to reduce the risk of cardiovascular events in patients at high cardiovascular risk [196].
Therefore, currently we recommend the use of omega-3 acids (in Poland Vazkepa is still unavailable, and combined formulations of omega-3 acids in a dose of less than 1 g are dominant) in treatment of hypertriglyceridaemia in a dose of at least 2 g daily, as adjunct treatment to statins and fibrates, except in patients already using omega-3 acids in combination with statins, in whom fibrates can be used as a 3rd line treatment.
KEY POINTS TO REMEMBER
Omega-3 polyunsaturated acids significantly reduce triglyceride concentration (by 20–30%) and hsCRP (by 12–20%).
In patients with hypertriglyceridaemia statins are the first-line agents. Addition of omega-3 acids in a dose of at least 2 g to a statin and a fibrate may be considered in patients with persistent hypertriglyceridaemia (TG > 200 mg/dl or 2.3 mmol/l) despite combination therapy.
If available, icosapent ethyl should be considered in a dose of 2 × 2 g in addition to a statin in very high-risk patients with ASCVD with persistent TG concentration > 150 mg/dl.
9.6. Bile acids sequestrants (resins)
Resins bind bile acids in the intestine, reducing their enterohepatic circulation. In this way, by feedback, conversion of cholesterol into bile acids in the liver is activated. Reduced cholesterol content in hepatocytes increases expression of the LDL receptor, which in turn reduces serum LDL-C concentration [197]. In some patients resins may increase hepatic VLDL formation, resulting in increased serum TG concentration. In addition, they reduce glucose concentration in patients with diabetes mellitus. Addition of colesevelam to antidiabetic agents significantly improves glycaemic control, although no data on the effect of such treatment on cardiovascular risk reduction are available [197].
Bile acids sequestrants in maximum doses, i.e., cholestyramine 24 g/day, colestipol 20 g/day, or colesevelam 4.5 g/day reduce LDL-C concentration by 18–25%. No significant effect on HDL-C levels has been observed. Treatment with cholestyramine in primary prevention in patients with hypercholesterolaemia resulted in significant reduction in cardiovascular episodes by 19% [198, 199]. A colesevelam preparation (Cholestagel) is available on the Polish market, and the use of ion exchange resins is currently limited to treatment of severe hypercholesterolaemia during pregnancy.
Resins are not absorbed from the gastrointestinal tract and demonstrate no systemic toxicity. However, they often cause gastrointestinal adverse effects (constipation, flatulence, nausea). They reduce absorption of fat-soluble vitamins. To avoid reduced absorption of other medicines, ion exchange resins should be taken 4 h before or 1 h after other medicines. Colesevelam is the best tolerated resin [200].
9.7. Nicotinic acid
Nicotinic acid (niacin) inhibits lipolysis in adipose tissue, thus reducing synthesis of free fatty acids (FFA) and their inflow into the liver [8, 201]. This leads to reduction of the amount of FFA supplied to the liver and therefore VLDL production. Reduced VLDL synthesis in turn leads to reduced production of intermediate-density lipoprotein (IDL) and LDL [8, 201]. In addition, niacin directly inhibits hepatic diacylglycerol O-acyltransferase 2 (DGAT2) – the key enzyme in triglyceride synthesis [8], and increases synthesis of apoA1 in the liver, leading to increase in HDL-C concentration [8, 201]. Nicotinic acid in a dose of 2 g/day reduces LDL-C concentration by ca. 15–18%, TG by ca. 20–40%, and Lp(a) by 30%, as well as increases HDL-C concentration by 25% [8, 201, 202].
Niacin is rarely used (in most countries it is unavailable or available in targeted import only) due to negative study results as well as adverse effects [8]. Results of the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) [203] and HPS2-THRIVE (The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) [204] studies contributed to a virtual lack of use of niacin in lipid-lowering therapy. In the AIM-HIGH trial, in high-risk patients with cardiovascular disease, addition of prolonged-release niacin (1500–2000 mg/dl) to standard statin therapy did not result in additional reduction of cardiovascular events (HR = 1.02; 95 CI: 0.87–1.21; p = 0.79), despite an increase of HDL-C concentration from 35 mg/dl (0.91 mmol/l) to 42 mg/dl (1.08 mmol/l), and a TG reduction from 164 mg/dl (1.85 mmol/l) to 122 mg/dl (1.38 mmol/l), Lp(a) from 36 to 27 nmol/l, and LDL-C from 74 mg/dl (1.91 mmol/l) to 62 mg/dl (1.60 mmol/l) [203]. Explanation of these results includes optimum treatment of ASCVD patients (during the study); what is interesting, despite the fact that in the niacin group almost twice as many patients had to reduce the dose because of adverse reactions (6.3%) and more patients discontinued treatment, the adherence was still above 75% in 90% of patients [203]. In the HPS2-THRIVE study, also no additional clinical benefit was observed from treatment with prolonged-release niacin and laropiprant (a compound that inhibits prostaglandin D2 synthesis responsible for skin flushing and hot flashes) in combination with a statin [204]. However, in the analysis of all components of the endpoints, significant reduction in coronary revascularisation and all revascularisation (10% reduction, p = 0.03) was observed in the niacin/laropiprant group. In this study, in comparison with statin monotherapy, significantly more cases of infection, hyperglycaemia, new cases of diabetes mellitus, gastrointestinal or musculoskeletal symptoms, gastrointestinal bleeding, and skin symptoms were noted. Those findings were surprising, given the earlier safety data for niacin; therefore, in comments following publication of the study, there was a suggestion that these adverse reactions could be largely due to the use of laropiprant [8, 9].
KEY POINTS TO REMEMBER
Currently, there are no indications for the use of nicotinic acid and no formulation of this agent is available in Poland.
In 2013, the EMA restricted the use of a slow-release formulation of nicotinic acid to the lipid disorders therapy with elevated triglyceride concentration, and only as an alternative therapy. At the same time, niacin in combination with laropiprant was withdrawn from the market.
In justified circumstances (in which the benefits outweigh the risks), nicotinic acid may be considered in order to reduce residual risk in patients with high Lp(a) concentration if other agents (i.e., PCSK9 inhibitors or inclisiran) are not available.
9.8. Combination therapy and guidelines concerning treatment of lipid disorders
In the context of potential combination therapy, we usually refer to high-risk and very high-risk patients. Unfortunately, more and more often we have problems with adequately effective treatment of low-risk patients, relatively young (40+, 50+), with one isolated risk factor, for example, elevated LDL-C concentration [2, 9]. In the European guidelines [8, 9], the management of this group has never been properly described from a practical point of view, i.e. what the non-pharmacological management should be, when to initiate pharmacological treatment, how to achieve the treatment goal effectively. Therefore, in reference to the ILEP recommendations [2], the authors of these guidelines point to the need of possibly optimum non-pharmacological management (lifestyle modification), and in case of failure (unfortunately, this may apply to up to 80% of individuals) [152], after not more than 6 months, to consider initiation of pharmacological treatment, i.e. nutraceuticals (preferably, in the form of polypills containing several natural substances with proven effect on LDL-C [135]), low-dose statin (with LDL-C reduction up to 30%), or ezetimibe (in case of statin intolerance), and if this is not effective, a combination of these therapies; the efficacy of this approach has been demonstrated in available studies (low-dose statin + nutraceutical, nutraceutical + ezetimibe) [135] (Figure 5).
Figure 5
Recommendations for low-risk patients with persistently elevated LDL cholesterol concentration (modified according to the ILEP 2020 recommendations [2])
Although many patients achieve their LDL cholesterol target concentration with monotherapy using appropriate doses, a large proportion of high-risk and very high-risk patients, or those with markedly elevated LDL-C concentration, require additional treatment. In the EUROASPIRE-V study, as much as 71% of high-risk patients did not achieve the treatment goal, and in the Da Vinci study, it was true for 2/3 of all patients, regardless of risk, and as much as 82% of very high-risk patients; it is also estimated that only ca. 8% of extreme risk patients achieve the treatment goal [30, 31, 205].
In such cases, combination therapy is justified and should be initiated as soon as possible, and in justified cases immediately after a diagnosis of lipid disorders was established. According to the ESC/EAS 2019 guidelines, addition of ezetimibe (as early as after 4–6 weeks) is recommended in very high-risk individuals and those at high risk despite statin therapy with maximum tolerable doses; if this is not sufficient to achieve treatment goals, addition of a PCSK9 inhibitor is recommended (after another 4–6 weeks). Therefore, the guidelines have definitely shortened the time in which our patient should receive combination therapy – to as little as 8 weeks. At the same time, for the first time (in line with the results of the EVOPACS, EVACS and VCU-alirocRT trials [179–181], the possibility of combination therapy with PCSK9 inhibitors during hospitalization was recommended. In most very high-risk patients this is the only chance to achieve the therapeutic goal, in accordance with the rules: “the lower the better”, but also “the earlier the better”. Therefore, the authors of these guidelines also recommend that in very high-risk patients (1) with baseline LDL-C concentration that prevents achievement of the therapeutic goal with statin monotherapy (e.g. in patients with LDL-C > 120 mg/dl (3.1 mmol/l), assuming that intensive treatment reduces LDL-C concentration by ca. 50%), (2) in those with extreme cardiovascular risk, (3) those with statin intolerance (complete or partial), and (4) in patients already receiving intensive statin treatment prior to hospitalisation, combination therapy with ezetimibe should be initiated immediately. Each patient group listed above should achieve the treatment goal as soon as possible, and LDL-C concentration should be as low as possible, even < 40 mg/dl (1 mmol/l) in patients with extreme cardiovascular risk. Patients with familial hypercholesterolaemia should also be mentioned here, in whom baseline LDL-C concentration may be above 300 mg/dl (7.8 mmol/l); in these patients only immediate initiation of triple therapy provides an opportunity to achieve the therapeutic goal (assuming 85% reduction on triple therapy). Detailed recommendations concerning the efficacy and use of combination therapy are presented in Tables XVII and XVIII, and in Figures 6–9. In this aspect, the authors of these guidelines agree with and adapt the recommendation of the International Lipid Expert Panel (ILEP) [109]. Of course, these recommendations still do not reflect actual circumstances, especially with respect to limitations concerning PCSK9 inhibitors, but provide a clear recommendation for immediate use of statin/ezetimibe combination therapy in selected groups of patients (similarly to lipid-lowering therapy) and indicate the need to extend the therapeutic programme with PCSK9 inhibitors as soon as possible, with the possibility of immediate inclusion for selected patient groups (i.e. without the need to wait 3-6 months, in accordance with current regulations).
Table XVII
Summary of recommendations on the principles of lipid-lowering therapy
Table XVIII
Recommendations on the intensity of lipid-lowering therapy including combination therapy depending on the cardiovascular risk categories
| Risk group | LDL-C | Non-HDL-C | Treatment |
|---|---|---|---|
| Extreme risk | < 40 mg/dl (1.0 mmol/l) | < 70 mg/dl (1.8 mmol/l) | Extremely intensive lipid-lowering therapy (%LDL-C reduction by 80–85%) Atorvastatin 40–80 mg/day + Alirocumab/Evolocumab Rosuvastatin 20–40 mg/day + Alirocumab/Evolocumab Atorvastatin 40–80 mg/day + Ezetimibe 10 mg/day + Alirocumab/Evolocumab Rosuvastatin 20–40 mg/day + Ezetimibe 10 mg/day + Alirocumab/Evolocumab Atorvastatin 40–80 mg/day + Inclisiran 300 mg every 3/6 months1 Rosuvastatin 20–40 mg/day + Inclisiran 300 mg every 3/6 months |
| Very high risk | < 55 mg/dl (1.4 mmol/l) and reduction of baseline LDL-C ≥ 50% | < 85 mg/dl (< 2.2 mmol/l) | Very intensive lipid-lowering therapy (%LDL-C reduction by 60–80%) Atorvastatin 40–80 mg/day + Ezetimibe 10 mg/day Rosuvastatin 20–40 mg/day + Ezetimibe 10 mg/day Atorvastatin 40–80 mg/day + Ezetimibe 10 mg/day + Bempedoic acid 180 mg/day2 Rosuvastatin 20–40 mg/day + Ezetimibe 10 mg/day + Bempedoic acid 180 mg/day Rosuvastatin 10 mg + Ezetimibe 10 mg/day + Bempedoic acid 180 mg/day Atorvastatin 20 mg + Ezetimibe 10 mg/day + Bempedoic acid 180 mg/day Alirocumab 150 mg every 2 weeks Evolocumab 140 mg every 2 weeks Rosuvastatin 5–10 mg/day (+ Ezetimibe 10 mg/day) + Alirocumab/Evolocumab/Inclisiran Atorvastatin 10–20 mg/day (+ Ezetimibe 10 mg/day) + Alirocumab/Evolocumab/Inclisiran Simvastatin 20–40 mg/day (+ Ezetimibe 10 mg/day) + Alirocumab/Evolocumab/Inclisiran |
| High risk | < 70 mg/dl (< 1.8 mmol/l) and reduction of baseline LDL-C ≥ 50% | < 100 mg/dl (< 2.5 mmol/l) | Intensive lipid-lowering therapy (%LDL-C reduction by 50–60%) Atorvastatin 40–80 mg/day Rosuvastatin 20–40 mg/day Rosuvastatin 5–10 mg/day + Ezetimibe 10 mg/day Atorvastatin 10–20 mg/day + Ezetimibe 10 mg/day Pitavastatin 4 mg + Ezetimibe 10 mg/day Simvastatin 20–40 mg/day + Ezetimibe 10 mg/day Pravastatin 40 mg/day + Ezetimibe 10 mg/day Lovastatin 40 mg/day + Ezetimibe 10 mg/day Fluvastatin 80 mg/day + Ezetimibe 10 mg/day Inclisiran 300 mg every 3/6 months |
| Moderate risk | < 100 mg/dl (< 2.5 mmol/l) | < 130 mg/dl (3.4 mmol/l) | Moderately intensive lipid-lowering therapy (%LDL-C reduction by 30–50%) Rosuvastatin 5–10 mg/day Atorvastatin 10–20 mg/day Pitavastatin 4 mg/day Simvastatin 20–40 mg/day Pravastatin 40 mg/day Lovastatin 40 mg/day Fluvastatin 80 mg/day Pitavastatin 1–2 mg + Ezetimibe 10 mg/day Simvastatin 10–20 mg/day + Ezetimibe 10 mg/day Pravastatin 20 mg/day + Ezetimibe 10 mg/day Lovastatin 20 mg + Ezetimibe 10 mg/day Fluvastatin 40 mg + Ezetimibe 10 mg/day Bempedoic acid 180 mg/day + Ezetimibe 10 mg/day |
| Low risk | < 115 mg/dl (< 3 mmol/l) | – | Low-intensity lipid-lowering therapy (%LDL-C reduction by < 30%) Pitavastatin 1 mg/day Simvastatin 10 mg/day Pravastatin 10–20 mg/day Lovastatin 10–20 mg/day Fluvastatin 40 mg/day Ezetimibe 10 mg/day Bempedoic acid 180 mg/day |
Figure 6
Algorithm for intensive lipid-lowering combination therapy in patients with ACS at very high or extreme risk
Figure 7
Algorithm for intensive lipid-lowering combination therapy in patients at extreme cardiovascular risk
Figure 9
Algorithm for intensive lipid-lowering combination therapy in patients with ACS optimally treated before hospitalization
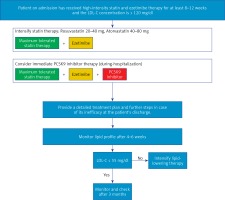
In addition, in some patients with mixed hyperlipidaemia (dyslipidaemia or atherogenic dyslipidaemia), in order to optimise cardiovascular risk, a fibrate (fenofibrate) or a formulation of unsaturated omega-3 acids should be used in addition to a statin or a statin and ezetimibe. The use of several agents with different mechanisms of action may significantly increase treatment efficacy, and in some cases makes it possible to use lower doses, which in turn leads to a lower risk of adverse reactions to these agents.
At the same time, it should be emphasised that lipid disorders are often accompanied by other conditions which often require pharmacotherapy. Therefore, patients treated for hyperlipidaemia often use several or even a dozen medicines at the same time, which results in errors, irregular medication use, and frequent discontinuation of treatment (i.e., the lack of adherence and/or compliance). For those reasons, in treatment of lipid disorders, as in treatment of arterial hypertension, combination preparations containing two or more active agents in one tablet are increasingly used. It was demonstrated that reduction of the number of tablets used and simplification of the dosing regimen, with the same daily doses of medicines used, is associated with more regular use of prescribed medication and less frequent treatment discontinuation, which directly translates into better treatment effects and, consequently, reduction of the risk of cardiovascular events [206, 207]. In treatment of hyperlipidaemia, combinations of different statins (atorvastatin and rosuvastatin in all doses) with ezetimibe in one tablet are currently available. Similarly, a combination of ezetimibe with bempedoic acid should appear on Polish market this year. It has been demonstrated that use of one preparation containing a statin and ezetimibe leads to greater reduction of cholesterol concentration and more frequent achievement of recommended cholesterol concentration than use of the same agents in the same doses, but as separate tablets [208, 209]. Results of studies demonstrating the efficacy and safety of combination formulations of bempedoic acid with ezetimibe as well as atorvastatin with fenofibrate are also available [209, 210]. Possible combinations of individual agents used in treatment of lipid disorders are summarised in Figure 10.
9.9. Recommendations on management of hypertriglyceridaemia
Hypertriglyceridaemia (HTG) is defined as fasting triglyceride (TG) concentration ≥ 1.7 mmol/l (150 mg/dl) and non-fasting ≥ 2 mmol/l (175 mg/dl). It may be mild to moderate with TG concentration of 1.7–9.9 mmol/l (150–885 mg/dl) or severe with TG concentration > 10 mmol/l (885 mg/dl); the latter is associated with a high risk of pancreatitis [211]. Mild to moderate HTG is related to elevated concentration of VLDL triglycerides (VLDL-TG) or triglyceride-rich lipoprotein (TRL) remnants, while in severe HTG, occurring much less often, chylomicrons in fasting plasma are present. HTG is classified as primary (Table XIX) or secondary (Table XX). Before treatment initiation, it should be diagnosed whether HTG is a primary disorder (occurring in only a few percent of patients) or is associated with another disease or medication. Primary hypertriglyceridaemia is a monogenic (rare) or polygenic (common) disorder [211]. Large population-based studies, clinical trials in secondary prevention, and genetic studies (variants of genes affecting TG concentration) have demonstrated an association between TG concentration and the risk of cardiovascular diseases [212]. Apparently, atherogenic properties are associated not as much with triglycerides themselves as with TG-containing lipoproteins, primarily smaller VLDL and so-called remnants, i.e., partially catabolised VLDL (largely free of triglycerides) and chylomicrons. Therefore, complex hyperlipidaemia (small VLDL + elevated LDL-C concentration) and dysbetalipoproteinaemia (remnants) are associated with a high risk of cardiovascular disease. The mechanism of atherogenic action of smaller VLDL and remnants is similar to that of LDL molecules. Newly formed chylomicrons themselves are not atherogenic because they are too large to enter the vascular wall. Thus, the main threat associated with severe HTG with fasting chylomicronaemia is acute pancreatitis (AP) [99, 213]. Up to 10% of AP cases develop as a consequence of severe HTG.
Table XIX
Classification of primary hypertriglyceridaemia
Table XX
Secondary causes of hypertriglyceridaemia
In patients diagnosed with hypertriglyceridaemia, secondary causes should be first ruled out, as appropriate management of a concomitant condition or modification of medications used may improve lipid profile. It should be noted that in secondary HTG indeterminated multigene genetic basis may also be present. In case of severe HTG, fasting serum is equally lipaemic (milky), and when stored in a refrigerator (temperature +4°C) for over 12 h, a layer of fat (chylomicrons) separates on the serum surface [99, 214]; this is a positive result of the cold flotation test (fridge test). Severe HTG with the presence of chylomicrons in fasting serum may be monogenic (very rarely) or polygenic (much more often) (Table XIX). Monogenic chylomicronaemia (formerly called familial chylomicronaemia syndrome, FCS or historically, according to the Fredrickson classification, type 1 hyperlipoproteinaemia) occurs with a prevalence of 1 case/100,000 population. Clinical signs, especially in homozygous individuals, include nodular xanthomatosis, yellow papules on the trunk, arms and lower extremities, and retinal lipaemia. In multifactorial or polygenic chylomicronaemia syndrome (MCS, or Fredrickson type 5 hyperlipoproteinaemia), in addition to chylomicrons, VLDL-TG concentration is also elevated. This lipid disorder is usually associated with factors increasing hypertriglyceridaemia, such as alcohol, carbohydrate-rich diet (fructose), uncontrolled diabetes mellitus, obesity, hypothyroidism, pregnancy, or certain medications [99].
In Table XIX, in addition to primary severe hypertriglyceridaemia, classification of mild to moderate hypertriglyceridaemia is presented. It includes multifactorial or polygenic HTG (formerly familial HTG or type 4 hyperlipoproteinaemia with increased VLDL-TG concentration), dysbetalipoproteinaemia (formerly type 3 hyperlipoproteinaemia or dysbetalipoproteinaemia or remnant disease) with elevated concentration of VLDL remnants and chylomicron remnants as a result of their impaired catabolism, and combined hyperlipoproteinaemia (formerly type 2b hyperlipoproteinaemia or familial combined hyperlipoproteinaemia) with elevated VLDL-TG and LDL-C concentration [212].
Although the target triglyceride concentrations have not been established, lower concentrations indicate lower cardiovascular risk and values > 2.3 mmol/l (200 mg/dl) have been considered an indication for pharmacological reduction [8, 9]. Failure to set the TG target results from the lack of evidence from randomised clinical trials that would make its determination possible. The most important treatment goal in prevention of cardiovascular diseases remains low LDL-C concentration, and in patients with TG concentration > 4.5 mmol/l (400 mg/dl), non-HDL-C concentration.
9.9.1. Dietary management
Dietary management is of significant importance in treatment of hypertriglyceridaemia [8, 9]. It may vary depending on whether the condition is a result of elevated concentration of VLDL triglycerides or chylomicron triglycerides and VLDL-TG. In patients with elevated VLDL-TG concentration, reduction and preferably avoidance of alcohol consumption is considered important. Obese patients should reduce body weight (improved sensitivity to insulin). Hyperinsulinaemia associated with abdominal obesity stimulates TG synthesis in the liver; lipolysis in adipose tissue is increased, and released fatty acids transported to the liver are a substrate for TG synthesis. Hypertriglyceridaemia may be a symptom of metabolic syndrome, in which abdominal obesity is usually the main feature. It may be said that obesity removes the mask of a patient with HTG. This also applies to alcohol and carbohydrate consumption [8, 9].
Important nutritional recommendations with high efficacy in reducing VLDL-TG include reduction of total carbohydrate intake, in particular mono- and disaccharides (fructose and sucrose). Carbohydrates are substrates for hepatic TG production. The effect of carbohydrate-rich products on triglycerides is much weaker if diet is based on high-fibre foods with low glycaemic index. In reduction of TG concentration, physical activity is also very important as working muscles use fatty acids contained in them as a source of energy [8, 9]. It must not be forgotten to replace saturated fats with mono-, and above all polyunsaturated fats [139, 143], or generally speaking animal fats with vegetable fats, with the exception of two tropical oils, i.e., coconut and palm oil.
In patients with elevated concentration of chylomicron triglycerides and VLDL triglycerides (polygenic chylomicronaemia), diet is very important, although more difficult to implement, as it should be targeted at reduction of chylomicron synthesis in the intestinal epithelium, so fat intake must be very limited (< 15–20% of energy) [99, 211, 213], and at the same time at reduction of VLDL triglyceride synthesis (recommendations discussed above). Chylomicrons are formed from both saturated and unsaturated fat, hence drastic reduction of total fat intake. The effect of such diet is very rapid. A large decrease in TG occurs after a few days. In some patients, medium-chain TG (MCT) may be considered as a source of energy; these are transported directly to the liver through the portal vein and metabolised there, so chylomicrons do not form. Alcohol abstinence is recommended. In monogenic chylomicronaemia (FCS), the primary treatment is low-fat diet, although recently a new agent has been introduced, which offers hope for effective treatment of patients with FCS.
9.9.2. Pharmacological management
In high-risk patients with TG concentration > 2.3 mmol/l (200 mg/dl), treatment is always initiated with a statin (atorvastatin or rosuvastatin). This is a class I recommendation. Following publication of the REDUCE IT study results, in which the use of EPA (icosapent ethyl 2 × 2 g/day) for 4.9 years in patients optimally treated with statins with fasting TG concentration 1.5 to 5.6 mmol/l (135–499 mg/dl) and high cardiovascular risk resulted in a reduction of incidence of cardiovascular events by 25% [147], European experts recommended adding EPA to a statin in such cases (IIaB) [9]. A fibrate may also be added to a statin in primary prevention (IIbB) as well as in high-risk patients in whom LDL-C concentration corresponds to the target and TG concentration exceeds 2.3 mmol/l (IIbC) [9]. The authors of these guidelines generally accept European recommendations, however, pointing out a much greater role of fibrates in high-risk patients, which may be very effective in reduction of the risk of micro- and macrovascular complications (recommendation level IIaB), and the fact that icosapent ethyl is still unavailable on Polish market; therefore, the recommendations include for the first time omega-3 acids in high doses (at least 2 g/day – recommendation level IIbC) (see sections on omega-3 acids and fibrates; Table XXI and Figure 11).
Table XXI
Recommendations on treatment of hypertriglyceridaemia
| Recommendation | Class | Level |
|---|---|---|
| Statins are recommended as first-line therapy to reduce the risk of CVD in high-risk individuals with hypertriglyceridaemia (TG > 2.3 mmol/l/> 200 mg/dl). | I | B |
| In at least high-risk patients with TG ≥ 1.7 mmol/l (≥ 150 mg/dl) despite statin treatment, icosapent ethyl (2 × 2 g/day) in combination with a statin should be considered.* | IIa | C |
| In at least high-risk patients with TG ≥ 2.3 mmol/l (≥ 200 mg/dl) despite statin therapy, omega-3 acids (PUFA in a dose of 2 to 4 g/day) in combination with a statin may be considered. | IIb | C |
| In patients in primary prevention who achieved their LDL-C goals with persistent TG concentration > 2.3 mmol/l (> 200 mg/dl), fenofibrate in combination with a statin may be considered. | IIb | B |
| In high-risk patients who achieved their LDL-C goals with persistent TG concentration > 2.3 mmol/l (> 200 mg/dl), fenofibrate in combination with a statin should be considered. | IIa | B |
Figure 11
Recommendations on treatment of hypertriglyceridaemia (adapted and modified, based on the EAS Expert Opinion 2021 [140])
If TG concentration is ≥ 5.6 mmol/l (500 mg/dl), treatment is initiated with fibrate to quickly decrease its concentration and reduce the risk of AP. If chylomicrons are present in the fasting state and VLDL-TG concentration is increased (multifactorial or polygenic chylomicronaemia), combination pharmacotherapy with a fibrate and n-3 PUFA (2 × 2 g/day) is used together with diet. In monogenic chylomicronaemia, the efficacy of treatment with a fibrate and PUFA n-3 is low, and as mentioned above, effective pharmacotherapy has become possible only recently [215]. It is also worth noting that recently (May 2019) the EMA has granted conditional approval for the use of a novel agent effectively lowering TG concentration in monogenic chylomicronaemia [215]. Volanesorsen is an antisense oligonucleotide that inhibits translation of apolipoprotein CIII (Apo CIII) mRNA. Apo CIII, present in lipoproteins transporting TG, inhibits lipoprotein lipase (LPL) activity. Volanesorsen is administered subcutaneously once a week for 3 months, then once every 2 weeks. It still has not been approved by the FDA. Thrombocytopenia is a common adverse reaction associated with volanesorsen (see section on new agents in treatment of lipid disorders) [215].
A practical summary of management of hypertriglyceridaemia is presented in Table XXII.
Table XXII
Summary of hypertriglyceridaemia management recommendations
9.10. New agents in lipid disorders therapy
9.10.1. Bempedoic acid
Bempedoic acid is an ATP-citrate lyase (ACL) inhibitor that decreases LDL-C concentration by means of inhibition of cholesterol synthesis in the liver. ACL is an enzyme preceding 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway [216]. Importantly, bempedoic acid is an inactive prodrug and requires activation by coenzyme A (CoA) with long-chain acyl-CoA 1 synthetase (ACSVL1), and the entire process takes place in the liver rather than in skeletal muscles, which from the very beginning indicated that it may be a very effective agent for statin-intolerant patients [216]. Inhibition of ACL by bempedoic acid decreases hepatic cholesterol synthesis and reduces blood LDL-C concentration by increasing the activity of LDL receptors; it also affects simultaneous inhibition of hepatic biosynthesis of fatty acids [216].
The efficacy of bempedoic acid has been investigated in numerous phase II studies and 4 pivotal phase III studies in the CLEAR programme (Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen). In the CLEAR Tranquility study [217], patients with a history of statin intolerance and LDL-C concentration ≥ 100 mg/dl were enrolled. After a 4-week lead-in period of treatment with ezetimibe, 269 patients were randomised 2 : 1 to bempedoic acid 180 mg or placebo once daily added to ezetimibe for 12 weeks. Bempedoic acid reduced LDL-C cholesterol by 28.5% in comparison with placebo (p < 0.001), as well as non-HDL (–23.6%), TC (–18.0%), ApoB (–19.3%), and what is particularly interesting in terms of residual risk associated with inflammation, hsCRP (–31.0%). The treatment was well tolerated; the incidence of treatment-related adverse events, muscle-related adverse events, and treatment discontinuation was similar between the bempedoic acid and placebo groups [217].
In the CLEAR Harmony study [218], patients with ASCVD and/or heterozygous FH and LDL-C concentration ≥ 70 mg/dl (1.8 mmol/l) despite maximum tolerated statin therapy with or without additional lipid-lowering therapy were enrolled. The primary endpoint was safety, and the key secondary endpoint was percent change in LDL cholesterol concentration after 12 weeks of treatment. The study included 2230 patients, of whom 1488 received bempedoic acid and 742 placebo; the follow-up period was 52 weeks. The incidence of adverse events and serious adverse events during the intervention period did not differ significantly between the two groups, but the incidence of adverse events leading to treatment discontinuation was higher in the bempedoic acid group than in the placebo group (162 (10.9%) patients vs. 53 (7.1%) patients), as was the incidence of gout (18 (1.2%) patients vs. 2 (0.3%) patients). Bempedoic acid reduced mean LDL-C concentration by 18.1% in comparison with placebo [218].
In the CLEAR Serenity study [219], 345 patients with hypercholesterolaemia and a history of intolerance to at least 2 statins, including one at the lowest available dose, were randomised. Mean age of the enrolled patients was 65.2 years, mean baseline LDL-C concentration was 157.6 mg/dl, and 93% of patients reported a history of muscle symptoms associated with statin therapy. Treatment with bempedoic acid, in comparison with placebo, significantly reduced LDL-C concentration by 21.4% (95% CI: –25.1 to –17.7%; p < 0.001), non-HDL-C by 17.9%, total cholesterol by 14.8%, as well as ApoB (–15.0%) and hsCRP (–24.3%). Bempedoic acid was safe and well tolerated; muscle-related adverse effects (muscle pain) occurred in 4.7% and 7.2% of patients who received bempedoic acid and placebo, respectively [219].
In another study, CLEAR Wisdom [220], a total of 779 patients with ASCVD and/or heterozygous familial hypercholesterolaemia and LDL-C concentration ≥ 70 mg/dl (1.8 mmol/l) while receiving maximum tolerated lipid-lowering therapy were enrolled. The follow-up period was 52 weeks. Bempedoic acid reduced LDL-C concentration significantly more than placebo (–17.4%; 95% CI: –21.0% to –13.9%; p < 0.001); a significant reduction of non-HDL cholesterol (–13.0%), total cholesterol (–11.2%), and ApoB concentration (–13.0%), as well as hsCRP (median: –18.7% vs. –9.4%), was also observed. Common adverse events included nasopharyngitis (5.2% vs. 5.1% for bempedoic acid and placebo, respectively), urinary tract infection (5.0% vs. 1.9%), and hyperuricaemia (4.2% vs. 1.9%) [220].
In another study, being a pooled analysis of 4 phase III studies, the efficacy and safety of bempedoic acid in patients with ASCVD and/or FH as well as in patients with partial and complete statin intolerance was assessed [221]. The final analysis included 2425 patients in the group receiving bempedoic acid and 1198 in the placebo group. Mean age of patients was 66 ±9 years and the mean baseline LDL-C value at randomisation was 108 ±33 mg/dl and 144 ±39 mg/dl in the statin-intolerant group. After 12 weeks of treatment, in comparison with placebo, LDL-C was reduced by 17.8% in the bempedoic acid group, whereas in the statin-intolerant subgroup, the LDL-C reduction was 24.5%; this effect was maintained after 52 weeks of the study. Significant decrease of concentration of non-HDL cholesterol, TC, ApoB, and hsCRP was also observed in the bempedoic acid group. Treatment with bempedoic acid was well tolerated but was associated with a higher incidence of mild (resolving upon treatment discontinuation) adverse events, such as increased uric acid concentration (2.1% vs. 0.5%), decreased eGFR (0.7% vs. < 0.1%), increased aminotransferase activity (2.8% vs. 1.3%), and an increased incidence of gout (1.4% vs. 0.4%) [221]. We have obtained very similar results in a meta-analysis of 10 phase II and III trials, which enrolled nearly 4000 patients, where, interestingly, as also observed in phase III trials, treatment with bempedoic acid was associated with a 41% reduction in the risk of development of new cases of diabetes (OR = 0.59; 95% CI: 0.39–0.90; p = 0.01) [222].
Only the CLEAR Outcomes study, in which 14,014 high-risk patients with statin intolerance were enrolled, will answer the question on how the addition of bempedoic acid may translate into a reduction in cardiovascular events. The study is planned to complete in December 2022; it will continue until 1620 patients experience the primary endpoint. The minimum duration of treatment will be 36 months, and the predicted median exposure to treatment – 42 months [223]. However, the currently available study results provided the basis for positive assessment of bempedoic acid and its combination with ezetimibe by the FDA (February 2020) and by the EMA (April 2020).
According to the EMA regulations, bempedoic acid is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia in addition to diet: (1) in combination with a statin or a statin and other lipid-lowering therapies in patients, in whom the target LDL-C reduction has not been achieved with the maximum tolerated statin dose, OR (2) alone or in combination with other lipid-lowering therapies in patients who are intolerant to or have contraindications for statin therapy. Based on available studies, the authors of these guidelines also decided to recommend bempedoic acid (and its combination with ezetimibe) in selected groups of patients with lipid disorders (Table XXIII).
Table XXIII
Recommendations on the use of bempedoic acid
9.10.2. Inclisiran
The primary mechanism of action of inclisiran is inhibition of PCSK9 synthesis (by catalytic degradation of PCSK9 mRNA), which binds to and promotes degradation of LDL-C receptors, resulting in increased LDL-C concentration. Inclisiran is a so-called short interfering RNA, i.e. a double-stranded RNA molecule with a length of about 20–25 base pairs that silences the expression of genes with homologous sequence (RNAi) – in this case the mRNA which carries information for PCSK9 synthesis [224]. It is worth remembering that for discovery of the phenomenon of RNA interference, American scientists Andrew Z. Fire and Craig C. Mello received a Nobel Prize in Medicine and Physiology in 2006.
Inclisiran binds to asialoglycoprotein receptors on the hepatocyte surface (present only on these cells) and then binds to the RNA-induced silencing complex (RISC) which has the activity of ribonuclease and makes it possible to degrade the information RNA (mRNA) coding for PCSK9. As a result, inclisiran decreases PCSK9 synthesis in ribosomes (by means of inhibition of translation, often referred to as “gene silencing”), increases the number of LDL receptors on the surface of hepatocytes, and decreases the LDL cholesterol concentration [225, 226]. Inclisiran is administered parenterally (subcutaneously) and its characteristic feature is a long duration of activity, which allows for application every 6 months.
The safety and efficacy of inclisiran is being evaluated in the ORION programme (and currently in the follow-up VICTORION programme), in which its efficacy in high cardiovascular risk patients with ASCVD or FH (both hetero- and homozygous) is evaluated, as well as in primary prevention in patients with so-called cardiovascular risk equivalent, and in populations with a so-called unmet need, i.e. patients with low treatment adherence (including statin intolerance), or those with chronic kidney disease (including those with severe renal impairment and GFR between < 30 ml/min/1.73 m2) and chronic liver disease [224–226].
In recently published studies, it has been demonstrated that inclisiran reduces LDL-C cholesterol by ca. 50% (up to 55%), among others in individuals with familial hypercholesterolaemia (the ORION-9 study, which enrolled 482 patients with FH and a mean LDL-C concentration of 4.0 mmol/l (155 mg/dl), 90% of subjects were treated with a statin and 53% with ezetimibe), those with a history of atherogenic cardiovascular disease (CHD, CVD, or PAD) (the ORION-10 study, which enrolled 1561 patients; mean LDL-C concentration 2.7 mmol/l, 89% treated with a statin, 10% with ezetimibe), and those with or without ASCVD, but with high cardiovascular risk, i.e. so-called ASCVD risk equivalent (the ORION 11 study, 1617 patients enrolled, mean LDL cholesterol concentration: 2.7 mmol/l, 95% treated with a statin, 7% with ezetimibe) [227, 228]. In addition, in these studies a decrease of triglyceride concentration by 7% to 13 %, and Lp(a) by 17% to 26% was observed, as well as an increase in HDL-C by 3% to 6% in the inclisiran-treated groups in comparison with patients receiving placebo. All studies demonstrated very good tolerance of the agent, except for local injection site reactions, which occurred several times more often in the inclisiran groups [227, 228].
The objective of the studies discussed above was not assessment the effect of inclisiran on the risk of cardiovascular events; therefore, the number of patients enrolled, and the number of such endpoints did not allow to draw reliable conclusions in this regard. However, a meta-analysis of these studies (total number of patients analysed: 3660) demonstrated that inclisiran not only reduced the LDL cholesterol concentration (by a mean of 50.5%) but also reduced the risk of major cardiovascular events (a composite endpoint of cardiac death, sudden cardiac arrest, myocardial infarction, and stroke) by 24% (p = 0.01) [229]. The meta-analysis once again confirmed very good tolerability of the product [229]. Furthermore, the association between decreased LDL-C concentration and reduced risk of cardiovascular events as a result of inclisiran treatment has been shown to be similar to the relationship between cardiovascular risk reduction and change in LDL-C concentration for alirocumab and evolocumab [230]. Of course, only the results of the ORION-4 study, which are awaited by the end of 2024, will answer the question on how this significant LDL-C reduction plus a unique mode of administration (two doses per year), which will definitely translate into improved adherence, will reduce cardiovascular events and mortality.
It is worth noting that completed phase II and III studies as well as pooled analyses of these studies and meta-analyses were the basis for the approval of inclisiran by the EMA in December 2020. According to this decision, the product should be administered in a dose of 300 mg subcutaneously (inclisiran sodium equivalent to 284 mg of inclisiran in 1.5 ml of solution); after the first injection, the next dose should be given after 3 months, and then every 6 months in the following indications:
in adults with primary hypercholesterolaemia (heterozygous familial hypercholesterolaemia and multigenic hypercholesterolaemia), or mixed dyslipidaemia, as an adjunct to diet,
in combination with a statin, or a statin with other lipid-lowering agents, in patients who cannot achieve their target LDL-C concentration with the maximum tolerated statin dose
OR
alone or in combination with other lipid-lowering agents in patients who are statin-intolerant or in whom statin treatment is contraindicated.
In Poland, inclisiran is available (few patients have been already administrated) but still not reimbursed, and work on the preparation of a therapeutic drug programme is ongoing. However, in view of the above, primarily the results of the available studies, the authors of these guidelines have decided to propose the first recommendations on the use of inclisiran and the groups of patients who might most benefit (Table XXIV).
Table XXIV
Recommendations on the use of inclisiran
9.10.3. Apabetalone
Apabetalone is an oral BET (bromodomain and extra-terminal domain) inhibitor with particular affinity for bromodomain-containing protein 4 (BRD4) [231]. Apabetalone binds to the second bromodomain, thus inhibiting epigenetic modulators of gene transcription. The effects of apabetalone include stimulation of gene expression and production of ApoA-I, the main component of high-density lipoproteins (HDL). Beneficial effects of the agent on severity of inflammation and the composition and volume of atherosclerotic plaques have also been demonstrated [231]. A meta-analysis of three small studies comprising the BETonMACE programme (n = 798) demonstrated a beneficial effect of the agent on the concentration of apolipoprotein A-I, HDL-C, the number of large HDL particles, and CRP [232]. In addition, a significant reduction of the risk of cardiovascular events in comparison with placebo was observed, especially in patients with diabetes, low HDL cholesterol concentration, and in those with elevated CRP concentration [232]. However, the published results of the BETonMACE study, which included 2425 post-ACS patients with type 2 diabetes and low HDL-C concentration, did not confirm a statistically significant difference in the risk of a composite endpoint of cardiovascular death, myocardial infarction, or stroke (10.3% vs. 12.4%, p = 0.11) between the groups receiving apabetalone (100 mg bid) and placebo [233]. As a result of good tolerability of the new agent and a low number of adverse reactions during treatment, results of subsequent studies on “the first agent modifying processes on the epigenetical level in patients with cardiovascular diseases” may be awaited; the agent may be a valuable addition to treatment of lipid disorders in selected groups of patients (currently, it seems that patients with atherogenic dyslipidaemia may comprise such a group) [231–233]. The results of a subanalysis of the BETonMACE study in patients with ACS, diabetes and chronic kidney disease may be a confirmation [234]. The median follow-up period was 27 months; in patients with CKD apabetalone in comparison with placebo was associated with fewer major adverse cardiovascular events (MACE) (HR = 0.50; 95% CI: 0.26–0.96) and hospitalisations related to heart failure (HR = 0.48; 95% CI: 0.26–0.86). In patients with CKD, a similar number of adverse events was observed, regardless of randomisation to apabetalone or placebo, and fewer serious adverse events (29% vs. 43%; p = 0.02) in the apabetalone group [234].
9.10.4. Volanesorsen
Volanesorsen is an antisense oligonucleotide that inhibits the synthesis of ApoC-III, a protein known as an inhibitor of lipoprotein lipase (LPL), a regulator of triglyceride metabolism and hepatic clearance of chylomicrons and other lipoproteins with a high content of triglycerides [235]. It has recently been shown that apoC-III increases triglyceride concentration on a pathway independent of lipoprotein lipase as well [236]. Volanesorsen selectively binds to information ribonucleic acid (mRNA) coding for apoC-III and prevents translation. The agent reduces the concentration of apoC-III by ca. 80–90% and that of triglycerides by ca. 70% [235]. The safety and efficacy of volanesorsen in patients with elevated triglyceride concentration were assessed in two phase III trials [236, 237]. The primary indication for volanesorsen is chylomicronaemia (FCS, type I hyperlipoproteinaemia).
In a recently published COMPASS study (phase III), adult patients (n = 114) with multifactorial severe hypertriglyceridaemia or FCS, BMI of 45 kg/m2 or less, and fasting plasma triglycerides at least 500 mg/dl were enrolled [238, 239]. Patients were randomised (2 : 1) to receive subcutaneous volanesorsen (300 mg) or placebo (1.5 ml) once a week for 26 weeks. After 13 weeks of treatment, the dose was changed to 300 mg of volanesorsen or placebo every 2 weeks. Volanesorsen reduced the mean plasma triglyceride concentration by 71.2% (95% CI: –79.3 to –63.2) from baseline, compared with 0.9% (–13.9 to 12.2) in the placebo group (p < 0.0001), which represents an absolute mean decrease of fasting plasma triglyceride concentration by 869 mg/dl (95% CI: –1,018 to –720; 9.82 mmol/l (–11.51 to –8.14)) in the volanesorsen group compared with a 74 mg/dl increase (–138 to 285; 0.83 mmol/l (–1.56 to 3.22); p < 0.0001) in the placebo group. In the safety analysis, 5 cases of acute pancreatitis were reported, all in the placebo group [239].
In August 2018, the FDA made a negative decision on the use of volanesorsen in FCS. In May 2019, volanesorsen (Waylivra) was approved by the EMA for the treatment of familial chylomicronaemia syndrome (FSC). It is available as a solution for subcutaneous injections, at the beginning of treatment administered once a week; after 3 months, patients in whom a sufficient decrease in triglyceride concentration has occurred may receive volanesorsen every 2 weeks. The frequency of injections is re-adjusted after 6 and 9 months of treatment.
9.10.5. Evinacumab
Evinacumab is a monoclonal antibody binding to angiopoietin-like protein 3 (ANGPTL3). The contribution of ANGPTL3 to lipid metabolism consists primarily in the inhibition of lipoprotein lipase (LPL) and endothelial lipase activity [240, 241]. In the phase III ELIPSE HoFH (Evinacumab Lipid Studies in Patients with Homozygous Familial Hypercholesterolemia) study, the use of evinacumab for 24 weeks was associated with a reduction in LDL cholesterol (baseline mean concentration of 255.1 mg/dl) by 49% (absolute reduction: 132.1 mg), and triglyceride concentration by 50% in patients with homozygous familial hypercholesterolaemia [240]. The agent is also effective in individuals with refractory hypercholesterolaemia. In a study involving 272 subjects (83% treated with a statin, 38% with ezetimibe, 96% with a PCSK-9 inhibitor) evinacumab reduced LDL-C concentration by 24% to 56%, depending on the dose and route of administration (300–450 mg/week, or 300 mg s.c. twice a week, or 15 mg/kg bw/4 weeks, or 5 mg/kg bw/4 weeks) [241]. The most recent analysis (a phase I study) demonstrated that the use of evinacumab in patients with mixed dyslipidaemia and elevated triglyceride concentration (even up to 1500 mg/dl) was associated with a very significant reduction of triglycerides, with a peak median reduction of 81.8% (compared with 20.6% in the placebo group); the median achieved concentration was 83 mg/dl vs. 444.0 mg/dl in the evolocumab and placebo group, respectively [242].
In February 2021, the FDA approved evinacumab (Evkeeza) as an add-on therapy for patients over 12 years of age with homozygous FH. The same recommendation was adopted by the EMA in June 2021. Evinacumab is administered as intravenous infusion over 60 min every 4 weeks in the recommended dose of 15 mg/kg body weight.
9.10.6. Antisense therapies
Translation is the second process (following transcription) in protein biosynthesis. Polypeptide chain formation is controlled by the mRNA sequence. Translation occurs in the cytoplasm or on the membrane of the endoplasmic reticulum. This process is catalysed by a ribosome as its subunits move along the mRNA strand [243]. In antisense therapy strategies, gene expression inhibition takes place at the stage of translation. In this method oligonucleotides with sequences complementary to mRNA areas critical for translation are used. These oligonucleotides bind to mRNA fragments and thus prevent protein synthesis [243]. An example of such an oligonucleotide is volanesorsen (discussed above). Another example is vupanorsen that inhibits ANGPTL-3 synthesis. Study results have demonstrated that the agent effectively reduces triglyceride concentration (by 36–53% depending on the dosage), while its effect on LDL-C concentration is relatively small [243]. Another agent, mipomersen, inhibits the synthesis of apolipoprotein B-100, one of the main molecules contained in low and very low-density lipoproteins (LDL and VLDL), and was registered in certain countries for the treatment of homozygous familial hypercholesterolaemia [244].
9.11. Management of elevated Lp(a) concentration
The molecule of lipoprotein (a) (Lp(a)), structurally similar to LDL, contains apolipoprotein (a) (apo(a)) which is covalently bound by a disulfide bridge to ApoB 100 [245, 246]. The apo(a) molecule is highly similar to plasminogen, which results in pro-atherogenic activity associated with the procoagulant effect. Due to its small diameter (< 70 nm), Lp(a) may flow freely across the endothelial barrier and, like LDL, be retained in the artery wall, which in turn may increase the risk of atherosclerotic cardiovascular diseases [246]. Pro-inflammatory and antifibrinolytic effects are also attributed to LP(a), which is important for progression of the atherosclerotic process [45, 246]. Large observational studies and meta-analyses have demonstrated a relationship between the Lp(a) concentration and the risk of ischaemic heart disease, ischemic stroke, and aortic valve stenosis [45, 247–250]. Although Lp(a) is a weaker risk factor than LDL-C, it is an independent factor, as evidenced by the fact that up to 30% of patients with familial hypercholesterolaemia and/or acute coronary syndrome may have an elevated concentration of this lipoprotein with a desired LDL-C concentration [45]. High Lp(a) concentration is associated with an increased risk of major cardiovascular events by 40% compared with low concentration [45]. A similar risk (41%) of ischaemic stroke was demonstrated in patients with Lp(a) > 50 mg/dl [9, 45].
The assumed desired Lp(a) concentration is < 30 mg/dl (< 75 nmol/l) (Table VIII). Conversely, a concentration ≥ 30 mg/dl indicates increased risk; it was assumed that concentrations > 180 mg (> 450 nmol/l) indicated a very high risk of myocardial infarction and aortic valve stenosis [9, 50, 249]. Detailed recommendations on when and in whom Lp(a) concentration should be measured have been discussed above in Sections 6.8 and 6.9, and Tables VIII and IX. Experts agree that at least once in every adult individual’s life Lp(a) concentration should be measured to detect patients at the highest risk, i.e., those with Lp(a) > 180 mg/dl. Furthermore, Lp(a) measurement should be considered in all patients with premature onset of cardiovascular disease, lack of effect of statin treatment, and in those at moderate to high risk. The authors of these guidelines also recommend consideration of Lp(a) measurement in individuals with ASCVD or FH, and in pregnant women. LP(a) has also been added to the definition of extreme risk patients as an additional risk-modifying factor in patients with ACS and diabetes (Table X).
Clinical trial results have demonstrated that lipid-lowering agents reduce Lp(a) concentration, although their effects are very variable (Table XXV). The most controversial results were obtained in patients treated with statins as both reduced and increased Lp(a) concentrations (particularly with pitavastatin) were observed [92]. Of currently available agents, the most promising clinical significance in Lp(a) reduction and incident reduction is attributed to PCSK9 inhibitors [251–253]. In the FOURIER study, in a group of patients with stable coronary artery disease treated with evolocumab, a 26.9% (6.2–46.7%) reduction of Lp(a) concentration was achieved, and a 23% incident reduction (HR = 0.77; 95% CI: 0.67–0.88) in those with baseline Lp(a) above the median (37 nmol/l ~15 mg/dl), while in the group with Lp(a) below the median by only 7% (HR = 0.93; 95% CI: 0.80–1.08) [252]. The number needed-to-treat (NNT) was 41 and 105, respectively. A significant relationship between a 15% reduction in the risk of major coronary events (95% CI: 2–25%; p = 0.0199) and a reduction of Lp(a) by 25 nmol/l was demonstrated after adjustment for LDL [252].
Table XXV
Effects of lipid-lowering drugs on Lp(a)
Similar results have been obtained in a subanalysis of the ODYSSEY OUTCOMES study in post-ACS patients treated with alirocumab. Risk reduction after 4 months of treatment analysed in patient groups with baseline Lp(a) concentration < 6,7 mg/dl, 6.7 to 21.2 mg/dl, 21.2 to 59.6 mg/dl, and ≥ 59.6 mg/dl was, respectively, 5% (HR = 0.95; 95% CI: 0.97–1.15), 15% (0.85; 0.71–1.03), 21% (0.79, 0.66–0.94), and 17% (0.83; 0.70–0.98). A reduction in Lp(a) by 5 mg/dl was associated with a significant reduction of cardiovascular events by 2.5% [253, 254]. Reduction of the risk of ischaemic heart disease was also demonstrated in an analysis of 62,240 patients with this disease compared with a control group of 127,000 patients. It was demonstrated that each reduction of Lp(a) by 10 mg/dl was associated with a reduction of the risk of ischaemic heart disease by 5.8% (OR = 0.94; 95% CI: 0.93–0.95). In contrast, a reduction of LDL-C concentration by 10 mg/dl resulted in a significant reduction of the risk of this disease by 14.5% (OR = 0.86; 95% CI: 0.82–0.89). It was demonstrated that with a decrease of Lp(a) concentration by 101.5 mg/dl a similar reduction in ischaemic disease was achieved as with a decrease of LDL-C by 38.7 mg/dl [255].
Significant reduction of Lp(a) concentration can also be achieved using lipoprotein apheresis or new agents (apo(a) antisense oligonucleotides, e.g., pelacarsen) which are still not widely available and require further clinical studies [45].
KEY POINTS TO REMEMBER
Lp(a) measurement is indicated at least once in every adult individual’s life to detect elevated lipoprotein (a) concentration that can significantly modify residual risk, as well as patients with the highest risk of ASCVD, i.e., those with Lp(a) > 180 mg/dl (> 450 nmol/l).
Measurement of Lp(a) should be considered in patients with premature onset of cardiovascular disease, lack of statin effect, and in those with a borderline risk level between moderate and high to improve risk assessment.
Measurement of Lp(a) may be considered in patients with very high cardiovascular risk and atherosclerotic cardiovascular disease, in patients with familial hypercholesterolaemia, and in pregnant women in prevention of pre-eclampsia or miscarriage, in recurrent pregnancy loss, or intrauterine growth restriction.
High Lp(a) concentration may cause an artifactual increase in LDL-C concentration.
9.12. The importance of antihyperglycaemic agents in treatment of lipid disorders and cardiovascular risk reduction
Pharmacological reduction of hyperglycaemia in multifactorial treatment of type 2 diabetes mellitus (in addition to treatment of hypertension and dyslipidaemia, lifestyle modification, antiplatelet therapy, etc.) is essential in prevention and inhibition of the progress of chronic diabetes-related complications (macro- and microvascular) and thus affects life expectancy [125, 256, 257]. In selection of therapy and combination of antihyperglycaemic medications, their effects on non-glycaemic parameters (mortality, cardiovascular or renal risk, body weight, risk of hypoglycaemia, lipid profile, etc.) should be taken into consideration while following the principle of personalisation of treatment. In patients with atherosclerotic cardiovascular disease, systolic heart failure, chronic kidney disease, or multiple cardiovascular risk factors, agents with proven beneficial effects on the risk of progression of these conditions as well as on overall and cardiovascular mortality should be used first (Table XXVI). This effect has been demonstrated for certain inhibitors of sodium-glucose cotransporter-2 (SGLT2) (flozins) and certain glucagon-like peptide-1 (GLP-1) receptor agonists [125, 256, 257]. In patients with chronic kidney disease and systolic heart failure, the choice of a flozin should be preferred, and if they are contraindicated, a GLP-1 analogue [125, 256, 257]. In patients diagnosed with ASCVD, both classes should be considered, and in the case of multiple risk factors, GLP-1 receptor agonists should be considered first. Early combination therapy with metformin and certain flozins and/or GLP-1 receptor agonists should be considered in the cases listed above in each patient, regardless of the achievement of the treatment goal [125, 256, 257]. Also in concomitant obesity, it is recommended to prefer GLP-1 receptor agonists or SGLT2 inhibitors. If the risk of hypoglycaemia is high, the same classes of agents and a dipeptidyl peptidase 4 (DPP-4) inhibitor or a peroxisome proliferator-activated receptors (PPAR-γ) agonist should be considered. In Poland, with limited reimbursement of new antihyperglycaemic agents, the most readily available and affordable classes of agents are sulfonylurea derivatives, PPAR-γ agonists, and acarbose [125, 256, 257].
Table XXVI
The effects of antihyperglycaemic agents on the lipid profile and cardiovascular risk
9.13. Apheresis in lipid disorders
9.13.1. LDL apheresis
LDL apheresis is a mechanical method of removal of LDL particles from serum. Blood collected from the patient is first divided in a separator into morphotic elements and plasma, which goes further into a set of LDL-C-separating filters. Once the plasma is filtered, it is transfused back to the patient together with cellular elements. The entire procedure lasts from 2 to 4 h. During this period, about 1.5–3 l of blood is filtered, and a reduction of LDL-C by 55–70% is achieved [258]. During apheresis, not only LDL-C, but also VLDL, fibrinogen, Lp(a), α2-macroglobulin, and coagulation factors are removed from the plasma [259]. Clinical observations suggest that long-term use of LDL-apheresis in patients with severe HoFH contributes to regression and stabilisation of atherosclerotic plaques, improves cardiovascular prognosis, and reduces xanthomata of the skin and tendons [260].
Despite high costs (the mean procedure cost amounts to PLN 5616) and the burden for the patient, LDL apheresis is still a very important complementary therapy for homozygous FH [259, 261–263]. The most recent ESC/EAS recommendations [9] and the position of the EAS experts [264] on HoFH did not significantly change the position on this issue, while recommending maintenance of pharmacological treatment at maximum tolerated doses [9, 264]. Importantly, LDL apheresis is a safe method for pregnant women [259, 261].
The results of key clinical trials which may significantly affect the position of LDL-apheresis in the next edition of recommendations are worth noting; even today, they are a real clinical alternative for the few patients undergoing these procedures in our country. The results of the TESLA [265] and TAUSSIG [266] studies concerning treatment of HoFH with evolocumab have demonstrated the efficacy of PCSK9 inhibitors in LDL-C reduction, comparable to LDL apheresis, with good treatment tolerance. Also in HeFH well-documented clinical trials have been performed and their results allow for replacement of apheresis with biological treatment. The ODYSSEY ESCAPE study met its primary endpoint showing that in patients in whom alirocumab was added to their previous regimen a significant 75% reduction in the frequency of apheresis in comparison with placebo was achieved. In 63% of patients receiving alirocumab apheresis was no longer required, compared with no such patients among those receiving placebo [267]. In view of lower costs and definitely better tolerability in comparison with LDL-apheresis, this creates a highly promising perspective for patients with HeFH. For patients with confirmed FH, such an alternative is already available in a therapeutic programme financed by the NHF (Table XVI).
In the position of the Working Group for Apheresis of the Polish Society of Nephrology [268] which was widely discussed and criticised at many sites, other (in addition to HoFH and HeFH) indications for treatment with LDL-apheresis have also been listed:
Primary prevention of cardiovascular disease: in patients with documented risk factors for coronary artery disease or its equivalent (e.g. peripheral atherosclerotic disease) who cannot be diagnosed with FH according to the Dutch criteria, although they have lipid disorders and do not achieve their LDL-C targets, according to the adopted guidelines (…), and in whom all other standard therapies have failed (for at least 3 months) or are poorly tolerated, and/or there are contraindications to pharmacological treatment (adverse effects, complications, e.g. rhabdomyolysis).
Secondary prevention of cardiovascular disease in high-risk patients diagnosed with cardiovascular disease (status post myocardial infarction or stroke, peripheral arterial disease), type 2 diabetes, or moderate to severe chronic kidney disease (CKD 4-5): in patients who cannot be diagnosed with FH according to Dutch criteria, although they have lipid disorders and do not achieve their LDL-C targets, according to the adopted guidelines (…), and in whom all other standard therapies have failed (for at least 3 months) or are poorly tolerated, and/or there are contraindications to pharmacological treatment (adverse effects, complications, e.g. rhabdomyolysis).
Isolated Lp(a) hyperlipoproteinaemia > 60 mg/dl with normal and/or high LDL-C concentration despite diet and maximum tolerated treatment for 3 months, with documented coronary artery disease.
Severe mixed hyperlipidaemia (refractory nephrotic syndrome in the course of focal segmental glomerulosclerosis).
Sudden sensory loss of hearing.
Severe hypertriglyceridaemia (TG ≥ 11.3 mmol/l (1000 mg/dl)) with acute pancreatitis with the use of double filtration LDL apheresis with citrate anticoagulation.
The most important adverse effects of LDL-apheresis include: hypotension, abdominal pain, nausea, vomiting, vertigo and headache, hypocalcaemia, iron deficiency anaemia, allergic reactions, haemolysis, and thrombocytopenia. Due to the risk of hypotension in patients treated for arterial hypertension, it is recommended to omit hypotensive medication on the day of the procedure. Complete blood count and iron concentration should be monitored and supplemented, if necessary [9]. Antiplatelet therapy should not be discontinued.
9.13.2. Apheresis in severe HTG
The procedure may be used in prevention of acute pancreatitis [269]. It is estimated that ca. 7% of cases of acute pancreatitis are associated with hypertriglyceridaemia [269]. The apheresis procedure may be considered on an individual basis, in addition to other elements of standard therapy [270], i.e., reduction of food energy and fat content, alcohol abstinence, and pharmacotherapy: fibrates (fenofibrate) and omega-3 fatty acids (2–4 g/day) (Sections 9.4 and 9.9). Effective insulin therapy is required in patients with diabetes. The efficacy of apheresis in acute pancreatitis has not been confirmed yet. The results of the only study comparing the efficacy of intensive insulin therapy with that of plasmapheresis are still unknown at the time of publication of these recommendations [271].
9.13.3. Lp(a) apheresis
The effects of reduction of Lp(a) concentration by means of apheresis have been documented not only in terms of anti-atherosclerotic, but also anti-inflammatory and anticoagulant activity; therefore, it is considered the intervention of choice in patients with high Lp(a) levels and signs of rapid progression of atherosclerosis [272]. German findings based on the German Lipoprotein Apheresis Registry (GLAR) demonstrated a 71% decrease in Lp(a) concentration with an associated decrease in MACE by 78% as early as after 2 years of follow-up [273]. As high efficacy of LDL-apheresis in reduction of Lp(a) concentration (> 60%) has been demonstrated, even in comparison with new treatment options (mipomersen ~25%, CETP inhibitors ~25%, PCSK9 inhibitors ~30%; Table XXV) it seems an interesting option for patients with high Lp(a) concentration and rapidly progressive atherosclerosis [274].
KEY POINTS TO REMEMBER
LDL-apheresis may be considered as adjunctive therapy in patients with HoFH.
LDL-apheresis should be considered in patients not meeting the criteria for treatment with PCSK9 inhibitors in therapeutic programmes (currently in HeFH and secondary prevention), when further progression of clinically evident atherosclerosis is observed despite maximum tolerated lipid-lowering therapy.
LDL apheresis should be considered in patients with high Lp(a) concentration and signs of rapid progression of atherosclerosis.
10. Treatment of lipid disorders in specific populations
10.1. Familial hypercholesterolaemia
Familial hypercholesterolaemia is a single-gene, autosomal dominant dyslipidaemia that results in life-long elevated serum LDL-C concentration, leading to premature complications of atherosclerosis. Untreated, it usually leads to premature CAD (in women before 60 years of age, and in men before 55 years) which means an up to 10-fold increase in the risk of CAD [275]. Heterozygous FH (HeFH) is relatively common; according to the latest meta-analysis including over 11 million patients, the rate for the world population is 1 : 313, but in patients with ischaemic heart disease the incidence is 10 times higher (1 : 31), with premature ischaemic heart disease 20 times (1 : 15), and in those with severe hypercholesterolaemia, 23 times higher (1 : 14) [276]. The global number of people affected by FH is estimated at 14–34 million [277], with only a small proportion of them diagnosed and treated [278].
In Poland, according to a meta-analysis of six large observational studies, based on the Dutch Lipid Clinic Network (DLCN) criteria (Table XXVII), FH was diagnosed in approximately one in 250 individuals aged 20–79 years [279], which translates into approximately 122.5 thousand people with FH in our country (based on the 2014 GUS data on the population of Poland). Similar estimates were obtained in other studies, although according to the LIPIDOGRAM study, which enrolled nearly 34,000 patients, the estimated prevalence may be even higher [278, 280].
Table XXVII
Diagnostic criteria for heterozygous familial hypercholesterolaemia (HeFH) according to the Dutch Lipid Clinic Network [8, 9]
Genetic causes of FH are single-gene loss of function mutations in the LDLR or ApoB genes or gain of function mutations in the PCSK9 gene. LDLR mutations are definitely most common (> 1700 different mutations have been identified [281]), while gain of function mutations in the PSCK9 gene comprise only a few percent of all FH cases.
In most cases, the diagnosis of FH is based on the clinical presentation, although significance of molecular testing is increasingly emphasised in the literature [282]. The superiority and importance of genetic testing consists primarily in the possibility of diagnosis at an early age by performing cascade diagnostics among first-degree relatives [9, 283, 284]. DLCN criteria, presented in the table above, are usually used in clinical diagnosis; alternatively, the Simone Broome registry or WHO criteria are used [8, 9]. It should be stressed that for proper assessment, one (the highest) criterion in each category (family history, clinical history, physical examination, LDL-C concentration, genetic testing) should be summed up. It is worth noting that LDL-C concentration should be measured without treatment; with statins, the values obtained may be multiplied by 1.43 [285] to estimate LDL-C concentration without a specific lipid-lowering therapy.
In the management of FH patients, effective treatment reducing LDL-C concentration (to the target values compliant with the ESC recommendations) [9] which may significantly reduce the risk of CAD is the most important issue. According to the criteria adopted in these guidelines, subjects with FH and without other major risk factors are considered high-risk patients, while those with FH and ASCVD or other major risk factors are considered very high-risk patients, which implies a recommendation to achieve specific treatment goals (< 55 mg/dl (1.4 mmol/l)). Furthermore, the authors of these guidelines believe that patients with FH and ACS should be considered extreme cardiovascular risk patients in whom, depending on baseline LDL-C values, immediate dual (intensive statin therapy + ezetimibe) or triple therapy (plus a PCSK9 inhibitor) should be considered (Tables V and XX, Section 9.8).
It is recommended to start treatment immediately once the diagnosis has been established. Modification of the patient’s lifestyle with respect to modifiable risk factors is a necessary but definitely insufficient therapeutic intervention. The treatment should include a potent high-dose statin, i.e., atorvastatin (40–80 mg/day) or rosuvastatin (20–40 mg/day), with a focus on the highest available doses of both statins. For very high-risk FH patients with ASCVD, the recommended treatment goal is reduction of LDL-C concentration by ≥ 50% from baseline and a target LDL-C concentration of < 1.4 mmol/l (< 55 mg/dl). Unless it is possible to achieve treatment goals with statin monotherapy, combination therapy with ezetimibe is recommended; this should be initiated immediately post diagnosis in selected patients (see above), with a focus on the role of combination tablets (polypills), further improving adherence to treatment.
In primary prevention in very high-risk patients with FH, reduction of LDL-C concentration by ≥ 50% from baseline and a target LDL-C concentration of < 1.4 mmol/l (< 55 mg/dl) should be considered the treatment goal. If this has not been achieved in very high-risk FH patients despite the use of the highest tolerated dose of a statin in combination with ezetimibe, a PCSK9 inhibitor is recommended (Tables XVII and XVIII).
Earlier than before, i.e., at the age of 5 years, it is recommended to start diagnostics for FH in children, and if HoFH is suspected, even earlier. That is why it seems so important to introduce the need for LDL-C measurement in the child’s health evaluation – at the age of 6 years at the latest. Unfortunately, the efforts to do so in Poland have not been successful so far. In children diagnosed with FH, it is recommended to start statin therapy at the age of 8, or at the latest 10 years, with education on appropriate diet. At the age > 10 years, the target LDL-C concentration should be < 3.4 mmol/l (< 130 mg/dl) [8, 9, 286]. The main problem is treatment of children with FH, since it is introduced gradually, usually too low doses are used, and it is often poorly monitored, which ultimately leads to very rare achievement of therapeutic goals in children [287].
Homozygous FH is a rare disease (ca. 1 : 160,000) resulting from the inheritance of a genetic mutation from both parents, resulting in pathologically elevated plasma LDL-C concentration (> 500 mg/dl) and an increased rate of atherosclerosis development (tendon and skin xanthomata below 10 years of age) and significantly increased cardiovascular risk [9, 265]. The prognosis in untreated HoFH is poor, and the majority of patients die before the age of 30 years. Since effective LDL-C reduction is the most important method to improve the prognosis in HoFH, intensive treatment should be carried out, involving all available interventions, i.e., high doses of potent statins, ezetimibe, PCSK9 inhibitors, and LDL apheresis [265, 284]. The ESC recommendations, as well as these guidelines, emphasise the importance of LDL apheresis [9], with the frequency adjusted to the patient’s individual needs. In this patient group, the efficacy of LDL-C reduction using PCSK9 inhibitors, i.e., evolocumab [265] and alirocumab [288], is well documented. Early genetic testing (including cascade screening of the patient’s relatives) and early intensive lipid-lowering therapy remain essential for the survival of patients with HoFH. Highly promising results have been achieved using new agents dedicated to this group of patients, including lomitapide (Lojuxta) [289] available in doses from 5 to 60 mg, mipomersen (Kynamro, which was not authorised for use by the EMA in 2013), as well as new therapies, including, above all, evinacumab (Evkeeza) (Section 9.10), which since June 2021, following a positive decision of the EMA, have been authorised for use in patients with HoFH in the European Union.
KEY POINTS TO REMEMBER
Heterozygous familial hypercholesterolaemia is a relatively common condition in Polish population with a prevalence of 1 case per 250 adults or higher (even up to 120-140,000 adult Poles).
In Poland, only ca. 5% of patients with FH have been diagnosed; most of them still remain undiagnosed and are not treated.
Genetic testing is highly useful in confirming the diagnosis of FH, especially in young patients and in screening of the family members (cascade screening), but is not required to initiate therapy;
Potent statins in the highest doses should be used in combination with ezetimibe; if therapeutic goals are not achieved, PCSK9 inhibitors should be added.
In extreme-risk patients (FH and ACS) and in those with high baseline LDL-C concentration (> 120 and > 300 mg/dl, respectively), immediate combination therapy with a statin and ezetimibe (polypill combination therapy is preferred) or triple therapy should be considered;
In primary prevention in very high-risk patients with FH and in patients with FH and ASCVD, the recommended treatment goal is reduction of LDL-C concentration by ≥ 50% from baseline and a target LDL-C concentration < 1.4 mmol/l (< 55 mg/dl).
10.2. Prediabetes and diabetes mellitus
Despite advances in early diagnosis and treatment strategies that reduce atherosclerotic CVD risk factors, diabetes mellitus remains one of the major causes of cardiovascular morbidity and mortality. It is an independent risk factor for CVD and nullifies the protective role of gender in women [290, 291]. Current data suggest that in patients with diabetes the risk of CVD is, on average, twice as high, but this risk varies widely depending on the population studied and the treatment applied. In patients with diabetes, increased cardiovascular risk is strongly determined by the presence of organ damage, mainly diabetic kidney disease. The diagnosis of type 2 diabetes typically coexists with other cardiovascular risk factors, such as abdominal obesity, dyslipidaemia, and arterial hypertension, defining so-called metabolic syndrome [290, 291].
A vast majority of patients with diabetes are at very high or high cardiovascular risk. The last guidelines [9], however, have definitely made risk stratification in patients with diabetes more difficult, which may translate into inappropriate treatment (underestimated risk resulting in not adequately intensive treatment) [292]. Therefore, the authors of these guidelines have decided to simplify risk assessment in patients with diabetes mellitus, considering that in each case at least high cardiovascular risk should be assumed (Table XXVIII).
Table XXVIII
Cardiovascular risk categories in patients with diabetes mellitus
| Extreme | Status post-acute coronary syndrome in a patient with diabetes and at least one additional risk factor (increased Lp(a) > 50 mg/dl or hsCRP > 3 mg/l or chronic kidney disease (eGFR < 60 ml/min/1.73 m2)) |
| Very high | Type 2 diabetes with organ damage1 or other major risk factors2,3, type 1 diabetes with early onset and duration > 20 years |
| High | Diabetes mellitus without organ damage (regardless of duration)4 |
10.2.1. Lipid disorders in patients with type 2 diabetes
For type 2 diabetes, atherogenic dyslipidaemia is characteristic, with elevated TG concentration, decreased HDL-C concentration, and usually increased LDL-C concentration in the lipid profile. It is a consequence of insulin resistance and relative insulin deficiency. Increasing concentration of large VLDL particles in type 2 diabetes initiates a sequence of events that generates atherogenic remnants, small dense LDL, and small dense dysfunctional HDL particles with high TG content [41]. In the natural course of type 2 diabetes mellitus, quantitative and qualitative changes of lipoproteins are observed already in prediabetes. Increased plasma concentration of Apo C-III slows down the removal of TG-rich particles. Thus, increased synthesis and defective catabolism of TG-rich particles lead to increased plasma concentration of TG and Apo B [293].
10.2.2. Lipid disorders in patients with type 1 diabetes
In individuals with type 1 diabetes and continuously well controlled glycaemia, a “super-normal” lipid profile is observed, characterised by high plasma HDL-C concentration as well as low TG and LDL-C concentration. In individuals with newly diagnosed type 1 diabetes, an increase of HDL-C concentration is observed as early as 3 months after initiation of insulin therapy [294]. This may be explained by subcutaneous administration of insulin, which increases LPL activity in adipose tissue and skeletal muscles, and consequently accelerates VLDL particle turnover [295]. A positive quantitative composition does not mean equally good quality and function. HDL-C dysfunctionality confers pro-atherogenic properties to these particles [296]. In a considerable proportion of patients with type 1 diabetes, concomitant obesity and insulin resistance-related metabolic disorders are observed. Too high doses of exogenous insulin and its route of administration generate secondary insulin resistance with its metabolic consequences.
10.2.3. Pharmacotherapy of lipid disorders in patients with diabetes
In patients with diabetes, the goal of lipid-lowering therapy is to reduce LDL-C concentration to values dependent on cardiovascular risk. The secondary target of therapy in this group is to reduce non-HDL-C concentration [9, 125]. In contrast, the authors of these guidelines are the first to point out that both LDL-C and non-HDL-C goals should be considered the primary objectives, which is particularly important for patients with atherogenic dyslipidaemia.
It is worth noting that adequate metabolic control of diabetes plays an important role in treatment of lipid disorders, especially in the case of hypertriglyceridaemia. Starting with the Heart Protection Study (HPS), research data suggest that all patients with type 2 diabetes benefit significantly from statin therapy (with a possible addition of ezetimibe), regardless of baseline LDL-C concentration [8, 9]. In the IMPROVE-IT study, in the subgroup of patients with diabetes, ezetimibe in addition to simvastatin was particularly effective, reducing the relative risk by 15% (95% CI: 6–22%), and the absolute risk by 5.5% [297]. The FOURIER study demonstrated that therapy with a PCSK9 inhibitor provides similar benefits in patients with and without diabetes; however, due to a higher baseline risk in patients with diabetes, a trend towards greater absolute risk reduction was observed (2.7% absolute reduction in major vascular events over 3 years) [298]. Of note, an LDL-C concentration of 0.8 mmol/l (31 mg/dl) was achieved in the evolocumab arm. The same benefits were demonstrated in the ODYSSEY Outcomes study in patients with diabetes mellitus post ACS [299]. Additional analyses concerning new agents, i.e., inclisiran and bempedoic acid, are also awaited. The latter may indeed be an interesting treatment option because it not only reduces LDL and non-HDL concentration, but also hsCRP, and the largest meta-analysis of Phase II and III studies has shown its significant efficacy in reducing the risk of new cases of diabetes [222] (Section 9.10).
There is evidence of an increased risk of diabetes mellitus with statin therapy, especially in patients with prediabetes or risk factors for diabetes, particularly those receiving intensive lipid-lowering therapy. Potential risk of diabetes should not affect the recommendation of statin therapy, as cardiovascular benefits should guide treatment strategies, and these are up to 5-fold higher than the risk of diabetes, particularly for atorvastatin and rosuvastatin [158]. A total lack of effect and even improved metabolic indices (glucose concentration, HbA1c, HOMA-IR) with pitavastatin treatment are also worth noting [150] (Section 9.1). Similarly, no increased risk of diabetes mellitus associated with the use of ezetimibe or PCSK9 inhibitors was observed in RCTs.
In type 2 diabetes, the lipid profile is often typical for atherogenic dyslipidaemia and, therefore, addition of fibrates to statin therapy may be associated with an additional benefit of reducing the incidence of cardiovascular events, but the evidence supporting recommendation of such management is not sufficient to date [9]. However, it should be emphasised that the evidence comes from studies with significant methodological limitations, and additional subgroup analyses confirmed the importance of treatment with fenofibrate in patients with diabetes. Moreover, the current ESC/EAS 2019 recommendations, approved also in these guidelines with the recommendation level increased to IIa (from IIb), unequivocally state that fibrate therapy should be considered in all statin-treated patients with hypertriglyceridaemia and TG concentration > 200 mg/dl (2.3 mmol/l). Subjects with type 1 diabetes with coexisting microalbuminuria and chronic kidney disease should be treated with statins regardless of baseline LDL-C values. Their goal should be reduction of LDL-C concentration by at least 50% from baseline [9] (Table XXIX).
Table XXIX
Recommendations on treatment of lipid disorders in patients with diabetes
10.3. Arterial hypertension and lipid disorders
Elevated arterial blood pressure and hypercholesterolaemia are, beside smoking, two main modifiable cardiovascular risk factors determining cardiovascular risk. An approach targeting both risk factors if they coexist is the basis for primary and secondary prevention of cardiovascular events.
In the WOBASZ II study, performed in the years 2013–2014 in a randomly selected cross-sectional sample of over 6000 individuals aged 19–99 years, the coexistence of arterial hypertension and hypercholesterolaemia in Polish population was assessed. In 34.5% of men and 31% of women (32.2% of the overall population), coexistence of these two main cardiovascular risk factors was found [300]. The prevalence of coexistence of lipid disorders and arterial hypertension depends on age. In the population of individuals aged 50–59 years, arterial hypertension and hypercholesterolaemia coexist in nearly half of the patients (46.2%). After 60 years of age, in more than 50% of the population lipid disorders coexist with arterial hypertension [300].
The WOBASZ study also made it possible to assess the frequency of control of arterial hypertension and lipid disorders [300]. The control rate of both arterial hypertension and lipid disorders in the overall population was 5.4% and in no age group, except for those aged 80 years and older, exceeded 10%. It should be noted that the low control rate could result from an unsatisfactory percentage of patients receiving pharmacological treatment – of patients with concomitant arterial hypertension and lipid disorders, only 59% received hypotensive treatment and only 31% lipid-lowering therapy. Factors associated with control of arterial hypertension and lipid disorders have also been identified. Multivariate analysis demonstrated that higher education and diagnosed cardiovascular disease were associated with achievement of therapeutic goals, whereas smoking was associated with worse control of arterial blood pressure and LDL-C concentration [300] (Section 13).
Treatment of arterial hypertension should be carried out in accordance with the Polish Society of Hypertension (PSH) 2019 guidelines, in which the 2018 ESC/ESH guidelines have been adapted. The need to use combination therapy, based on fixed-dose combinations (a two-component product in the 1st step of the therapy and a three-component product in the 2nd step), and to achieve lower arterial blood pressure values than previously accepted (i.e., 120–129/70–79 mm Hg) in patients below 65 years of age, should be emphasised [301, 302]. It has been demonstrated that simplified therapy with the use of fixed-dose combination products is associated with improved compliance [303]. Therefore, combination products containing antihypertensive agent(s) and a statin are a valuable supplement to the therapy. Combination products available in Poland containing two antihypertensive agents and a statin are based on optimum and consistent with the guidelines combinations of long-acting antihypertensive agents and a potent, long-acting statin; therefore, they can be used once daily in the morning [301].
Special populations should also be taken into consideration, which should be more often and more closely than individuals in the general population controlled for risk factors, including arterial blood pressure and lipid profile parameters:
patients with arterial hypertension and target organ damage (left ventricular hypertrophy, moderate albuminuria) [301, 302, 304],
women with a history of pre-eclampsia or gestational hypertension [305],
young people with isolated systolic hypertension [306],
patients with obstructive sleep apnoea [306],
patients with primary hyperaldosteronism [306],
patients with atherosclerotic renal artery stenosis [306].
The patient groups listed above are at increased cardiovascular risk; therefore, therapeutic interventions should be earlier and more intensive in these groups.
KEY POINTS TO REMEMBER
Coexistence of arterial hypertension and hypercholesterolaemia is very common. The level of control of arterial hypertension and hypercholesterolaemia is definitely too low.
Treatment of arterial hypertension is based on combination therapy with fixed-dose combination products. Combination products containing an anti-hypertensive agent (or agents) and a statin are available; their use may lead to improved control of arterial hypertension and hypercholesterolaemia by simplifying therapy and increasing compliance (adherence).
Several groups of patients have been identified which, due to their cardiovascular risk being higher than that assessed using classic risk scores, require careful control of arterial blood pressure and cholesterol concentration, as well as earlier and more intensive therapeutic decisions, e.g. patients with arterial hypertension and target organ damage, women with a history of gestation-related hypertensive states, young individuals with isolated systolic hypertension, and patients with secondary forms of arterial hypertension.
10.4. Ischaemic heart disease
10.4.1. Stable coronary syndromes
All patients with documented coronary atherosclerosis are at very high cardiovascular risk or extreme cardiovascular risk as defined previously. The rules for management of lipid disorders in this group of patients remain the same as in other patients at very high and/or extreme risk. In patients at very high cardiovascular risk, the treatment goal is to reduce LDL-C concentration by ≥ 50% from baseline and achieve a target LDL-C concentration of < 1.4 mmol/l (< 55 mg/dl). In patients at extreme cardiovascular risk, reduction of LDL-C concentration by ≥ 50% from baseline should also be aimed at (although not considered the treatment goal), with a target concentration of < 40 mg/dl (1 mmol/l) (Tables X and XI).
The mainstay of treatment are potent statins (atorvastatin and rosuvastatin), administered in high doses, allowing for the above-mentioned reduction by ≥ 50% and achievement of the treatment goals (Table XVIII). In patients undergoing coronary angioplasty (PCI) or coronary artery bypass grafting (CABG), administration of a loading statin dose before the planned procedure should be considered, and the treatment goals remain the same as discussed above.
Despite their high efficacy, even with the most potent statins used in monotherapy the patients are less and less likely to achieve their target lipid concentrations (at present, the proportion does not exceed 40%) [179]. If high-intensity statin therapy remains ineffective, combination therapy with agents of a different mechanism of action should always be considered. The primary agent used in combination treatment is ezetimibe which has already been available for 4 years in the form of generic products and combination products with statins (polypills). If combination treatment with a statin and ezetimibe remains ineffective, PCSK9 inhibitors should be added. In case of intolerance of high-dose statins, a low dose of a statin should be used in combination with other agents. Atorvastatin and rosuvastatin may also be used every 2–3 days with significant reduction of LDL-C concentration [307]. In case of complete statin intolerance, treatment with ezetimibe, bempedoic acid, or PCSK9 inhibitors//inclisiran, or even nutraceuticals as monotherapy or in combination therapy, should be considered.
In the current guidelines [9], much lower LDL-C target concentrations in comparison with the previous guidelines should be noticed. This position was based on the results of trials in which combinations of statins with ezetimibe, or statins with PCSK9 inhibitors and/or ezetimibe were used. Historically, the first large study in patients with recent ACS who received more intensive lipid-lowering therapy with simvastatin and ezetimibe (IMPROVE-IT) demonstrated significantly higher efficacy of combination therapy and improved long-term outcomes in comparison with statin treatment alone [297]. In the 7-year follow-up period, long-term maintenance of low LDL-C concentration (< 55 mg/dl (< 1.4 mmol/l)) was not associated with any obvious adverse effects [297]. New recommendations were affected by even better outcomes of LDL-C lowering therapies that have been achieved with addition of PCSK9 inhibitors to conventional treatment. In combination with high or maximum tolerated statin doses and/or ezetimibe, alirocumab and evolocumab reduced LDL-C concentration by 46–73% in comparison with placebo and by 30% in comparison with ezetimibe [308]. In patients who cannot use statins, PCSK9 inhibitors administered in combination with ezetimibe reduce LDL-C concentration by more than 60% and significantly reduce atherosclerotic plaque volume [309]. Both alirocumab and evolocumab have been shown to effectively reduce LDL-C concentration in patients at high and very high (as well as extreme) cardiovascular risk, including those with diabetes, inflammation, hyper-Lp(a), peripheral vascular disease/multiple level atherosclerosis, after several vascular events, post-stroke, and the elderly [49]. In addition, it was found that maintenance of low LDL-C concentration (even < 20 mg/dl (< 0.5 mmol/l)) for several years did not cause any worsening of cognitive function or a higher risk of dementia in treated individuals, and even led to a reduction in all-cause mortality and a significant reduction in further cardiovascular events [310].
The algorithms for management of patients with chronic stable coronary syndromes are presented in Table XXX.
Table XXX
Recommendations for target LDL cholesterol values in patients with stable coronary syndrome at very high or extreme risk
| Recommendations | Class | Level |
|---|---|---|
| In secondary prevention patients at very high risk it is recommended to reduce LDL-C concentration by ≥ 50% from baseline1 with LDL-C concentration of < 1.4 mmol/l (< 55 mg/dl) recommended as the target value. | I | A |
| In patients (1) with ASCVD who had a second vascular event within 2 years (not necessarily of the same type as the first), (2) after ACS and with peripheral vascular disease or polyvascular disease2 (multilevel atherosclerosis), (3) post ACS with multivessel coronary disease, (4) post ACS with familial hypercholesterolaemia, and (5) post ACS in a patient with diabetes and at least one additional risk factor (elevated Lp(a) > 50 mg/dl or hsCRP > 3 mg/l or chronic kidney disease (eGFR < 60 ml/min/1.73 m2)) despite maximum tolerated statin therapy, LDL-C concentration < 1.0 mmol/l (< 40 mg/dl) may be considered the target value. | IIb | B |
| Routine pre-treatment or loading (in patients receiving chronic statins) with a high dose of statin should be considered in patients undergoing PCI for ACS or elective PCI. | IIa | B |
1 The term “baseline” refers to LDL-C concentration in a person not receiving any LDL-C-lowering therapy. In individuals receiving an agent (agents) that reduce LDL-C concentration, predicted baseline LDL-C concentration (without treatment) should be estimated on the basis of the average efficacy of a specific agent or a combination of agents with respect to LDL-C reduction;
2 Polyvascular disease (= multilevel atherosclerosis) is defined as the presence of significant atherosclerotic lesions in at least two of the three vascular beds, i.e. coronary vessels. cerebral arteries, and/or peripheral vessels. ASCVD – atherosclerotic cardiovascular disease, LDL-C – low density lipoprotein cholesterol.
KEY POINTS TO REMEMBER
Statins are the first-line treatment in patients with stable coronary syndrome.
In every patient, and primarily after PCI or CABG, one should aim to achieve LDL-C concentration < 1.4 mmol/l (< 55 mg/dl).
In treatment of a patient after percutaneous coronary intervention, with regard to LDL-C concentration, the rules of “the lower the better”, “the earlier the better”, and “the longer the better” should be applied.
In each patient planned for PCI or CABG, a loading dose of a potent statin should be considered.
In each patient meeting the definition of extreme cardiovascular risk, one should aim to achieve LDL-C concentration < 1.0 mmol/l (< 40 mg/dl).
After percutaneous coronary intervention, each patient should undergo lifelong lipid-lowering therapy.
A large percentage of patients after percutaneous coronary intervention require combination treatment; in some of them it should be initiated already during hospitalisation (Section 9.8) in order to achieve the treatment goal.
Fixed combination products (polypills) available on the market are very helpful in treatment, mainly as a tool to improve the patient’s therapy adherence.
10.4.2. Acute coronary syndromes
Following an acute coronary syndrome (ACS), patients are at increased risk of recurrent cardiovascular events, which in Poland may affect up to 20% of patients within 1 year after the incident. In all ACS patients without contraindications or intolerance to statins, treatment with a potent statin in a high dose (atorvastatin 80 or rosuvastatin 40 mg daily) is recommended, i.e. should be continued or initiated as soon as possible, regardless of baseline LDL-C concentration. If the target LDL-C concentration has not been achieved after 4–6 weeks of statin therapy at the highest tolerated dose, it is recommended to start combination therapy with a statin and ezetimibe. If the target LDL-C value has not been achieved after another 4–6 weeks, addition of a PCSK9 inhibitor is recommended. It means that treatment with PCSK9 inhibitors can be initiated as early as after 8 weeks. In patients who develop ACS and have not achieved their target LDL-C concentration despite the use of a statin in the highest tolerated dose in combination with ezetimibe, addition of a PCSK9 inhibitor immediately after the event (if possible, even during hospitalisation) should be considered. Treatment with ezetimibe in combination with a statin during hospitalisation is currently the subject of a vigorous debate. Although no trials are available to support the clinical efficacy of this therapy, based on the rules of the lower the better and the earlier the LDL-C goal is achieved the better, the authors of these guidelines recommend that combination therapy with a statin and ezetimibe may be considered during hospitalisation, in particular in patients (1) already receiving intensive/optimal therapy, (2) in statin-treated patients with still high LDL-C concentration (> 100 mg/dl), (3) in untreated patients with baseline LDL-C concentration too high to achieve their target LDL-C concentration after 4–6 weeks of statin treatment (> 120 mg/dl), (4) in extreme-risk patients, and (5) in patients with partial or complete statin intolerance (Table XXXI, Section 9.8, Figures 6–9).
Table XXXI
Recommendations for lipid-lowering therapy in patients with acute coronary syndromes (ACS)
As in patients with stable coronary syndrome, in those undergoing percutaneous coronary intervention for ACS, routine initial treatment or loading (in patients receiving chronic statins) with a high dose of statin should be considered. Such treatment in ACS reduces infarction size [311]. Initial treatment with a statin also reduces the risk of contrast-induced acute kidney injury after coronary angiography or PCI.
If a statin-based regimen is not tolerated at any dose (even after rechallenge), the use of ezetimibe in monotherapy or in combination with PCSK9 inhibitors should be considered [312]. The algorithms for management of patients with myocardial infarction, including those with extreme cardiovascular risk, are presented in Figures 6–9.
KEY POINTS TO REMEMBER
In each patient with acute coronary syndrome, the maximum tolerated statin dose should be initiated as soon as possible, regardless of the lipid profile.
In each patient with acute coronary syndrome, administration of a loading dose of a potent statin before PCI should be considered.
In each patient post-acute coronary syndrome, one should aim to achieve LDL-C concentration < 1.4 mmol/l (< 55 mg/dl) as early as possible to effectively prevent further events.
In each patient meeting the definition of extreme cardiovascular risk, the treatment goal should be LDL-C concentration < 1.0 mmol/l (< 40 mg/dl).
Lipid-lowering therapy in each post-ACS patient should be lifelong.
A large proportion of post-ACS patients require combination therapy to achieve their treatment goal.
Fixed combination products (polypills) available on the market are helpful in treatment, mainly as a tool to improve the patient’s therapy adherence.
10.5. Cerebral stroke
The use of statins in primary prevention makes it possible to reduce the risk of ischaemic stroke by 22% [313]. Patients with a history of stroke or a transient ischaemic attack (TIA) are at a higher risk of another cerebral event (by as much as 40%), but also of other major cardiovascular events [9]. Patients with atherosclerotic stroke require intensive lipid-lowering therapy, all the more so that the results of a meta-analysis of randomised trials involving more than 170,000 patients indicate that reduction of LDL-C concentration by 1.0 mmol/l (38 mg/dl) with statins decreases the risk of serious vascular events (myocardial infarction, coronary death, all-cause stroke, or coronary revascularization) by about 22%, and all strokes by 17% [159]. Post-stroke patients should be treated as those with very high or extreme cardiovascular risk (as patients with multibed disease), and the treatment goal should be reduction of LDL-C concentration by ≥ 50% from baseline and achievement of the target LDL-C concentration of < 55 mg/dl (< 1.4 mmol/l) or < 40 mg/dl (1 mmol/l), respectively (Tables X and XI).
Individuals with haemorrhagic stroke often do not benefit from lipid-lowering therapy, and it may even be harmful if this form of stroke occurs in non-atherosclerotic patients [314]. However, it should be noted in this context that the lack of benefit in patients post haemorrhagic stroke is a different issue from the risk of stroke with intensive lipid-lowering therapy (statins or combination therapy, including PCSK9 inhibitors) and even extremely low LDL-C values achieved (< 20 mg/dl). Available data from recent meta-analyses of randomised trials and observational studies do not confirm such an association [315–317].
KEY POINTS TO REMEMBER
In primary prevention of stroke, statin therapy should be recommended in patients with other atherosclerotic diseases.
In patients with a history of atherosclerotic stroke or TIA, intensive statin therapy or intensive lipid-lowering combination therapy are the mainstay of treatment; the aim is to achieve the treatment LDL-C goal as soon as possible.
No causal relationship between intensive lipid lowering therapy (statins, combination therapy including PCSK9 inhibitors) and in consequence very low LDL-C concentration, and the risk of haemorrhagic stroke has been confirmed.
10.6. Peripheral vascular disease
Atherosclerotic lesions are the predominant cause (> 95%) of chronic lower limb ischaemia and amputation. Symptoms of lower limb ischaemia in the form of intermittent claudication may sometimes be the first clinical manifestation of systemic atherosclerosis [9]. Peripheral arterial atherosclerotic lesions are an independent risk factor for cardiovascular events, including ACS and stroke. To improve prognosis, in a patient with peripheral arterial atherosclerosis active pharmacological and non-pharmacological management should be urgently initiated [10]. In this group of patients, lipid-lowering therapy not only contributes to inhibition of atherosclerosis progression in the peripheral arterial bed, but reduces the risk of serious events in other vascular beds (i.e., coronary, cerebral) [9]. That is why not only peripheral vascular disease, but multibed disease, defined as the involvement of at least two out of three vascular beds, has been recently discussed. Especially now, in the era of innovative therapies, analyses are available indicating that intensive lipid-lowering therapy, especially combination therapy with the use of PCSK9 inhibitors, may translate into a highly significant reduction in the risk of patients with multibed disease, and the more advanced the disease (more beds involved), the greater the benefits. Data concerning alirocumab indicate that such treatment may translate into an absolute risk reduction by up to 13%, with the benefit seen in every 7–8 patient (NNT = 8) [113].
A meta-analysis of 18 clinical trials involving more than 10,000 patients with lower limb atherosclerosis has demonstrated that lipid-lowering therapy decreases the risk of cardiovascular events by nearly 20% and reduces all-cause mortality by 14% [314]. Patients with peripheral atherosclerotic disease (multibed disease) should be treated as patients with very high or extreme cardiovascular risk, and the treatment goal should be reduction of LDL-C concentration by ≥ 50% from baseline and achievement of the target LDL-C concentration of < 55 mg/dl (< 1.4 mmol/l) or < 40 mg/dl (1 mmol/l), respectively (Tables X and XI).
KEY POINTS TO REMEMBER
In patients with peripheral atherosclerosis, treatment goals and management are the same as in other patients with very high/extreme cardiovascular risk.
In patients with peripheral vascular disease, non-pharmacological lifestyle modification is essential, including non-smoking and increased regular physical activity (Table XV, Section 8.5), including march training (which not only has a positive effect on lipoprotein concentration, but stimulates development of collateral circulation and improves metabolism of ischaemic muscles).
In a patient with risk factors and cardiovascular disease, one should always bear in mind the assessment and monitoring of peripheral vascular bed for atherosclerotic lesions (ankle-brachial index (ABI), Doppler ultrasonography).
10.7. Heart failure
Numerous available studies have demonstrated that treatment with statins in patients with ischaemic heart disease as well as in primary prevention reduces the risk of heart failure [8, 9, 318]. It has also been demonstrated that high-dose statins used in these groups reduce the risk of hospitalisation due to heart failure in comparison with low doses [318]. Patients with advanced chronic heart failure usually have lower cholesterol concentrations [9, 318]. In contrast to those without heart failure, low cholesterol concentration in patients with heart failure is associated with poorer prognosis. Although the results of observational studies suggest a beneficial effect of statins on the risk of death in patients with heart failure, this effect has not been confirmed in interventional studies (despite numerous methodological errors of these studies). However, the safety of statins in patients with heart failure has been demonstrated [318–321].
It has been suggested that the role of lipid-lowering therapy may vary slightly depending on the left ventricular systolic function, which may be due to different aetiology and different mechanisms leading to development of heart failure [320–322]. Opinions based on analysis of pathogenetic mechanisms of heart failure and the mechanism of action of statins, but not the results of large clinical trials, indicate potentially greater benefits in patients with heart failure with preserved ejection fraction. Statin therapy has not been demonstrated to reduce the risk of death in patients with heart failure with reduced ejection fraction; however, a meta-analysis of 12 placebo-controlled randomised trials indicates that statin therapy may be associated with a 12% reduction in the risk of hospitalisation due to heart failure (confidence interval: 8–16%) [323]. No results from randomised trials are available to evaluate the efficacy of statins in patients with heart failure with preserved ejection fraction. However, analyses of observational studies suggest that expectation of such benefits is reasonable [320].
In summary, according to current evidence, statins are not recommended when heart failure is the only indication. However, it seems reasonable to continue statin therapy in patients who develop ischaemic heart failure. An indirect comparison of the efficacy of lipophilic and hydrophilic statins in patients with heart failure indicates lower risk of cardiovascular events in the group receiving lipophilic statins (atorvastatin, pitavastatin, simvastatin) than in the hydrophilic rosuvastatin group [324]. At present, still no data on the efficacy of PCSK-9 inhibitors in patients with heart failure are available. Treatment with unsaturated omega-3 acids may bring little benefit, as has been demonstrated in the GISSI-HF study (a reduction in the risk of death by 9%) [325], although the study included a relatively small number of patients with heart failure of any aetiology, and only 1 g of a mixture of omega-3 acids daily was applied, which, in view of our current knowledge, is an ineffective dose in terms of achieving a significant reduction of cardiovascular events (currently at least 2 g daily is recommended, with the target of 4 g) [325] (Tables XIII and XIV, Sections 8.4 and 9.9).
KEY POINTS TO REMEMBER
Lipid-lowering therapy with statins should not be applied if heart failure is the only indication.
Statin therapy should be continued in patients with ischaemic heart disease who develop heart failure. Dyslipidemic therapy discontinuation is one of the most common errors observed in the therapy of patients with heart failure.
10.8. Chronic kidney disease
In patients with chronic kidney disease, early evaluation of the complete lipid profile is recommended. In these patients, atherogenic dyslipidaemia is usually observed, often with normal or slightly elevated LDL-C and elevated Lp(a) concentration [326]. Cardiovascular risk categorisation is based on the stage of chronic kidney disease, cholesterol concentration, and other clinical and demographic characteristics. Individuals with advanced chronic kidney disease are at very high (eGFR < 30 ml/min/1.73 m2) or high (eGFR 30–60 ml/min/1.73 m2) cardiovascular risk (Table V).
In patients with chronic kidney disease, direct relationship between cholesterol concentration and cardiovascular risk is less pronounced than in general population [327]. The results of a meta-analysis of 28 randomised trials indicate that relative benefits of lipid-lowering therapy decrease with progression of chronic kidney disease. The relative risk of a vascular event associated with a reduction of LDL-C concentration by 1 mmol/l with a statin is 0.78 (95% CI: 0.75–0.82) in patients with eGFR ≥ 60 ml/min/1.73 m2 and 0.76 (0.70–0.81), 0.85 (0.75–0.96), 0.85 (0.71–1.02), and 0.94 (0.79–1.11) in those with eGFR in the range of 45–60 ml/min/1.73 m2, 30–45 ml/min/1.73 m2, < 30 ml/min/1.73 m2 not receiving dialysis therapy, and those receiving dialysis therapy, respectively (p for trend 0.008) [328]. Similar results have been obtained by other authors, indicating no benefit in patients with end-stage renal disease and in those receiving dialysis [329], no or minor effect on specific parameters of renal function (depending on treatment duration), and decreased effect of reduction of specific lipid fractions in this group of patients [330, 331]. This can be explained in a number of ways, one of which is the lack of real possibility of statin effect due to increased inflammation and vascular calcification; it is also worth mentioning that (severe) chronic kidney disease so strongly modifies cardiovascular risk that it is no longer possible to significantly reduce this risk with statin treatment.
Similar relationships are observed when considering the association of statin use with the risk of other endpoints, including all-cause mortality. This may be due to relatively higher non-vascular mortality in patients with more advanced renal disease, as well as difficulties in correct diagnosis of vascular events due to their atypical symptoms in patients with kidney failure [332]. As mentioned above, no effect of lipid-lowering therapy on prognosis in patients receiving dialysis therapy has been demonstrated, whereas available evidence justifies the recommendation of statins in kidney transplant patients [333].
Ezetimibe in combination with a statin reduced the risk of cardiovascular events in patients with chronic kidney disease [334], although the SHARP study did not provide clear answers, despite a demonstrated 17% reduction in the primary endpoint. In the study, methodological errors were made, consisting in modification of the endpoint during the study (so-called major atherosclerotic events were assessed), or the lack of a control group, i.e. individuals receiving statin monotherapy; therefore, it is difficult to draw conclusions from the results of this study alone [335].
It has been demonstrated that in selected groups of patients with chronic kidney disease, fibrate therapy may reduce the risk of cardiovascular events, but not all-cause mortality [336]. However, while statins have beneficial effects on glomerular filtration and proteinuria, the use of fibrates may be associated with increased creatinine concentration [336]. High efficacy of PCSK9 inhibitors in terms of lowering LDL-C concentration and in reducing the risk of cardiovascular events in patients with chronic kidney disease (with eGFR ≥ 30 ml/min/1.73 m2) has been demonstrated, similar to their efficacy in other patient groups [337, 338]. Interestingly, studies with inclisiran suggest that this may be the first lipid-lowering therapy that can be used in patients with end-stage renal disease with eGFR 15–30 ml/min/1.73 m2 [339].
The safety of lipid-lowering therapy is particularly important in advanced stages of chronic kidney disease. The risk of adverse events depends on blood concentration of the agent or its metabolites, affected by both the dose and renal function. In patients with chronic kidney disease, increased risk of drug interactions is observed. It is reasonable to prefer agents that are predominantly metabolised and eliminated by the liver (atorvastatin, fluvastatin, pitavastatin, ezetimibe) [340]. In certain studies, comparing the efficacy and safety of atorvastatin and rosuvastatin in patients with chronic kidney disease, more favourable effects of atorvastatin have been demonstrated [341].
In general, the target LDL cholesterol concentration in patients with chronic kidney disease does not differ from that in other patient groups and depends primarily on the cardiovascular risk category. Due to safety concerns, gradual escalation of lipid-lowering therapy should be considered, especially in patients with advanced chronic kidney disease [340]. First-choice lipid lowering agents in patients with chronic kidney disease should be statins. Certain analyses suggest that in this class of agents, only atorvastatin and rosuvastatin have proven effect on the risk of cardiovascular events in people with advanced chronic kidney disease [342]. In addition, atorvastatin less often requires dose adjustment due to renal function. Concerns about safety of the applied treatment may justify the preference of low-dose statin therapy combined with ezetimibe over high-dose statin monotherapy [9]. Concomitant use of statins and fibrates in patients with chronic kidney disease is not recommended [340]. It should be emphasised that available data are still insufficient, and recommendations are based on just a few large, randomised trials, meta-analyses, and post-hoc analyses of subgroups of patients in large clinical trials.
In conclusion, patients with advanced chronic kidney disease are at very high (those with eGFR < 30 ml/min/1.73 m2) or high (eGFR 30–60 ml/min/1.73 m2) cardiovascular risk. Intensive lipid-lowering therapy is recommended in patients not requiring dialysis. Statins are first-choice agents; combination therapy with ezetimibe and PCSK9 inhibitors should be considered in patients who do not achieve their treatment goal. In patients without atherosclerotic cardiovascular diseases who receive dialysis therapy, lipid-lowering agents should be initiated with great caution or not initiated at all (Table XXXII).
Table XXXII
Recommendations on treatment of lipid disorders in patients with chronic kidney disease
10.9. Children and adolescents
With respect to children and adolescents, no credible data on the efficacy and safety of long-term treatment of lipid disorders are available. There is a common belief that treatment should be started in childhood, but the time point of treatment initiation is usually arbitrary [343]. Delaying appropriate treatment until adult age, especially in children at risk, can lead to development of cardiovascular disease at an early age or in young adults. Recommendations with this regard are largely extrapolated from studies on familial hypercholesterolaemia and from studies in adults [343].
The aim of treatment of dyslipidaemia in children and adolescents is to achieve LDL-C concentration < 130 mg/dl (< 3.4 mmol/l, below the 95th percentile) or its reduction by 30–50%. In children with diabetes mellitus or FH and a family history of coronary artery disease before 40 years of age, it is recommended to achieve LDL-C concentration < 100 mg/dl (< 2.6 mmol/l) or its reduction by at least 50% [344–346]. So far, no similar cut-off points have been established for non-HDL-C or TG.
Treatment of dyslipidaemia in children and adolescents involves broad health education, lifestyle modification, and pharmacotherapy. Education should apply to all members of the child’s family. The scope and method of provided information should take into account the child’s age and the effectiveness of communication with caregivers. It is necessary to plan enough time to provide information and, if necessary, to complete this task even during numerous visits. Information should concern the causes and consequences of lipid disorders, cardiovascular risk factors, and principles and importance of therapy. Personalised counselling should be applied, and views, concerns and doubts of the young patient and their family should be openly discussed. Decisions concerning management should also be made collectively. It is reasonable to minimise the risk of providing contradictory advice, especially by doctors and nurses of the same practice, but also by specialist medical staff. The educational tasks are facilitated by previously developed, appropriate therapeutic relationships and awareness of psychosocial circumstances, both those facilitating (e.g., positive emotions, high social status) and hindering lifestyle modification (stress, tension in the family, social isolation) [8, 344, 345]. Lifestyle modifications include the introduction and consolidation of healthy dietary habits, focus on increasing physical activity, normalisation of body weight, prevention of passive smoking, and smoking and alcohol abstinence in adolescents. Lifestyle modifications should be introduced as early as at 2 years of age in every child with LDL-C concentration > 100 mg/dl (2.6 mmol/l) and/or elevated TG concentration (children < 10 years with TG ≥ 100 mg/dl (≥ 1.1 mmol/l); children 10-19 years with TG ≥ 130 mg/dl [1.5 mmol/l]) [344, 345] (Table XXXIII).
Table XXXIII
Recommendations concerning lifestyle changes in children with lipid disorders
Dietary management should be initiated in every child with dyslipidaemia above 2 years of age. Dietary interventions at an earlier age should be introduced by an experienced physician at a specialist clinic, with the assistance of a dietician. If possible, a dietician should be involved in the entire treatment process, especially as all dietary changes require careful monitoring of the child’s development [347]. If the effect of dietary treatment supervised by the family physician is insufficient, the patient and the family should be referred for dietary consultation (which extends beyond the care guaranteed by the NHF) or to a specialist clinic (cardiology, metabolic diseases) that provides such services. Elevated LDL-C concentration is an indication for: reduction of the energy supply from fats to 30%, including < 7% from saturated fats (the less the better), replacement of saturated fats with unsaturated fats (primarily polyunsaturated), and reduction of cholesterol intake < 200 mg/day [9, 348] (Section 8). Clinical studies have confirmed normal development of children undergoing dietary fat restriction provided that their needs for energy and micronutrients are met [9, 111, 348, 349]. Increased dietary fibre intake is recommended (in the amount of about 10 g at 5 years of age, 15 g at 10, and 20 g at the age of 15), as well as consumption of sea fish, vegetables, fruits, nuts and seeds, skimmed milk, and introduction of plant sterols and stanols (up to 2 g/day) [345, 350]. Increased TG concentration is an indication to reduce the consumption of monosaccharides, to increase the intake of fibre and polysaccharides, and to reduce body weight [351].
Parents should be motivated to significantly limit or prohibit watching TV by children under 2 years of age. Older children should be advised to limit the time spent before a TV or computer screen to 2 h/day, if possible, and to increase their physical activity to 90 min of exercise per day [347, 352].
Pharmacotherapy should be initiated after 6 months of lifestyle modification if treatment goals have not been achieved. Its long-term efficacy and safety have not been established. Statins are well tolerated; especially as very high doses are seldom used in the paediatric population [353]. The values at which pharmacotherapy is recommended in children and adolescents ≥ 10 years of age are presented in Table XXXIV.
Table XXXIV
Initiation of pharmacotherapy in children and adolescents, risk factors and lipid concentration
| Patient characteristics | Lipid parameter and concentration |
|---|---|
| No cardiovascular risk factors | LDL-C ≥ 190 mg/dl (4.9 mmol/l) |
| With one high1 risk factor and two intermediate2 risk factors, with a family history of early cardiovascular disease (before 55 years of age) | LDL-C ≥ 160 mg/dl (4.2 mmol/l) |
| With diabetes or with FH | LDL-C ≥ 130 mg/dl (3.4 mmol/l) |
| Without or with risk factors | TG ≥ 200 mg/dl (2.2 mmol/l) |
Statin therapy may be initiated by a family physician in children ≥ 10 years of age (except for children with FH, in whom the Forum of Lipid Experts in Poland recommends the use of statins > 8 years of age (accumulating data support lowering of this age even to 6 years), and in children with homozygous FH < 8 years of age with LDL-C concentration > 500 mg/dl (12.9 mmol/l)) [344]. Results of two measurements (performed 2 weeks to 3 months apart) in the fasting state and assessment of cardiovascular risk factors should be taken into account. Treatment starts with the lowest available dose, administered once daily in the evening [344]. The dose should be increased slowly, depending on the therapeutic effect, and the occurrence of possible adverse reactions should be monitored. The activity of aminotransferases and creatine kinase should be assessed prior to treatment [8, 344, 354]. Treatment with ezetimibe should be initiated under the supervision of a physician at a specialist clinic. The safety and efficacy of this agent in patients under the age of 17 have not been established, although there is also no evidence of any risk associated with such treatment. No precise dosing recommendations are available; in this case, based on data for the adult population, a dose of 10 mg/day should be suggested. Principles of the use of new therapeutic options, i.e., mipomersen [355] or PCSK9 inhibitors, have not yet been established in children, although in treatment of familial hypercholesterolaemia, these agents provide some hope for the future, especially when studies with alirocumab and evolocumab have been completed in children with both homo- and heterozygous FH. Available results from the Odyssey KIDS and HAUSER-RCT studies indicate the safety of PCSK9 inhibitors in the paediatric population and high efficacy (LDL-C reduction from 44.5 to 46%) [356, 357]. In addition, studies with inclisiran in children with FH (ORION 13 and 16) were also initiated. Dosage of lipid-lowering agents in children as well as adverse effects and contraindications are presented in Table XXXV. If the target LDL-C concentration has not been achieved with lifestyle modification and maximum statin doses, combination of lipid-lowering agents may be considered [358].
Table XXXV
Agents used in treatment of lipid disorders in children and adolescents available in Poland
In case of hypertriglyceridaemia, pharmacotherapy is usually reserved for patients with a high TG concentration (> 500 mg/dl – risk of acute pancreatitis, urgent reduction required) and genetic diseases (Section 9.9). The child should be referred to a specialist clinic for detailed diagnostics of elevated triglyceride concentration, and the possibility of treatment with statins, fibrates and omega-3 fatty acids should be considered [359, 360].
KEY POINTS TO REMEMBER
Treatment of lipid disorders should be initiated in childhood, since delaying therapy to adulthood may lead to development of cardiovascular diseases as early at a young age.
The aim of treatment of dyslipidaemia in children and adolescents is to achieve an LDL-C concentration < 130 mg/dl (< 3.4 mmol/l) and in children with diabetes mellitus or FH < 100 mg/dl (< 2.6 mmol/l) or at least a 50% reduction in LDL-C.
Health education should be provided to the patient as well as all his/her family members. Lifestyle recommendations include similar elements as in adults; other modifiable risk factors should also be considered when introducing them; dietary treatment should be initiated by the family physician only in a child over 2 years of age.
Pharmacotherapy should be initiated after 6 months of lifestyle modification if treatment goals have not been achieved; statins can be introduced in children ≥ 10 years of age (> 8 years in children with heterozygous familial hypercholesterolaemia).
10.10. Elderly individuals
Cholesterol is a significant risk factor for coronary artery disease, regardless of age, although this relationship is somewhat less pronounced in the elderly. A reduction in TG concentration by 1 mmol/l (38.7 mg/dl) is associated with a reduction in cardiovascular mortality in patients aged 40–49 by about 50%, and in patients aged 80–89 by only 15% (HR = 0.85) [361, 362].
The most important way to prevent cardiovascular diseases in the elderly is to promote a healthy lifestyle. A meta-analysis of 28 randomised clinical trials in patients over 75 years of age demonstrated that statin therapy reduced the relative risk of major cardiovascular events by 21% (RR = 0.79, 95% CI: 0.77–0.81) for each 1.0 mmol/l of reduction in LDL-C concentration [363]. These benefits were independent of age for individuals diagnosed with cardiovascular disease (also in the oldest population) but decreased with age in those receiving statins in primary prevention and no significance for individuals older than 70 years of age was shown. In the same study, a 12% (RR = 0.88, 95% CI: 0.85–0.91) reduction in the risk of cardiovascular death for every 1 mmol/l of reduction in LDL-C concentration was also observed [363]. Another meta-analysis confirmed these results not only for statins, but also for non-statin agents, showing a significant reduction in the risk of major vascular events for all assessed endpoints, regardless of age. However, it should be noted that also in this analysis, individuals in secondary prevention comprised a majority [364]. A valuable supplement to the results discussed above is the latest meta-analysis of 10 observational studies which included over 815,000 patients aged over 65 years in primary prevention [365]. This analysis is the more valuable because in randomised trials primarily composite endpoints are assessed rather than the effect of a specific therapy on the components of these endpoints; usually, the number of elderly patients is very limited, not to mention the follow-up duration, usually up to 5 years (in this analysis, the follow-up ranged from 5 to 24 years) [365]. The authors demonstrated that statin therapy in the elderly was associated with a significant 14% reduction in all-cause mortality, a 20% reduction in cardiovascular mortality, a 15% reduction in stroke, and a 26% numerical reduction (not statistically significant) in the risk of myocardial infarction. Importantly, this significant effect (reduction of all-cause mortality) was maintained regardless of age, also in patients > 75, > 80, and > 85 years of age (risk reduction of 12, 16, and 12%, respectively), in both women and men, but mainly in individuals with diabetes (18% risk reduction) [365].
The most recent ESC/EAS guidelines (2019) on the management of lipid disorders recommend that treatment with statins in primary prevention in individuals ≤ 75 years of age be used according to the estimated level of cardiovascular risk (IA). After 75 years of age, statin treatment in primary prevention may be considered in high- or very high-risk individuals (IIb B) [9]. In secondary prevention, statin treatment is recommended in elderly patients diagnosed with cardiovascular disease, according to the same rules as in younger patients (IA) [9].
Old age is a factor causing significant changes in pharmacokinetics, mainly at the stage of distribution (increased content of adipose tissue and α1 acid glycoprotein, reduced water content and albumin concentration) and elimination (impaired renal function, slower hepatic metabolism) [153, 366]. In addition, treatment in this group of patients is complicated by multimorbidity, the need of polypharmacotherapy, and patient non-compliance. Old age is an independent factor of increased risk of statin intolerance, especially muscle complaints [153]. Therefore, the International Lipid Expert Panel recommends treatment of the elderly with hydrophilic statins (rosuvastatin, pravastatin), as it is associated with greater safety [153]. Statin therapy should be initiated with low doses, gradually increasing them to achieve the target LDL-C concentration [8, 9]. Temporary discontinuation of a statin should be considered in elderly patients in situations in which there is an increased risk of intolerance, e.g., hypothyroidism, acute severe infection, major surgery, or malnutrition, bearing in mind that discontinuation of therapy increases both general and cardiovascular mortality [153] (Table XXXVI).
Table XXXVI
Recommendations on treatment of lipid disorders in the elderly
10.11. Autoimmune, rheumatic, and inflammatory diseases
In the course of autoimmune, rheumatic and inflammatory diseases, an increased risk of cardiovascular diseases is observed [8, 367]. Increased cardiovascular risk in diseases such as systemic lupus erythematosus, psoriasis, psoriatic arthritis, antiphospholipid syndrome, rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis, or Crohn’s disease is associated with vasculitis and endothelial dysfunction, leading to aggravation of atherosclerosis [8, 368]. This results in higher rates of cardiovascular morbidity and mortality in individuals suffering from these diseases in comparison with general population [8, 369]. It should be emphasised that currently there are no indications for the preventive use of lipid-lowering agents solely on the basis of the presence of autoimmune diseases, rheumatic diseases, or diseases of inflammatory aetiology, and prevention and treatment of dyslipidaemia does not differ from general rules of management in this regard. However, it is worth remembering that in the case of autoimmune, rheumatic, or inflammatory diseases, the values of lipid parameters may increase as a result of anti-inflammatory treatment of these diseases [369]. It is also worth noting that in this patient population, lipid-lowering therapy may be difficult due to elevated creatine kinase (CK) activity; therefore, the therapy should be monitored, in close contact with the attending physician (rheumatologist or gastroenterologist). In such cases, a combination therapy (with low-dose statins) or even the use of non-statin lipid-lowering agents may be considered (depending on the risk and target LDL-C values).
KEY POINTS TO REMEMBER
Autoimmune, rheumatic, and inflammatory diseases are associated with aggravation of atherosclerosis resulting in increased cardiovascular morbidity and mortality.
Before initiating treatment of dyslipidaemia in individuals with autoimmune and rheumatic diseases, it should be borne in mind that the classical use of the SCORE to assess cardiovascular risk in these patients may not be sufficient and the actual risk may be higher than estimated.
Prevention and treatment of dyslipidaemia in patients with autoimmune, rheumatic, and inflammatory diseases does not differ from general rules of management in this regard.
It should be remembered that lipid-lowering therapy may be difficult due to elevated CK activity and higher risk of statin intolerance; therefore, combination therapy may be considered in these patients, and therapy should be performed in cooperation with the attending physician.
10.12. Pregnancy and lactation
During pregnancy, the greatest challenge associated with potential lipid disorders is significant up to 2.5× physiological increase in triglycerides in the second, and in particular the third trimester of pregnancy, which may be associated with a higher risk of pancreatitis. Total cholesterol and LDL-C concentration usually increase by not more than 50%, but a significant (30–40%) increase in lipoprotein(a) concentration may also be observed which may increase the risk of pre-eclampsia, premature delivery, or low birth weight [370].
Statins should be discontinued for at least 3 months before planned pregnancy, as well as during pregnancy and lactation [8]. Statins may have teratogenic properties and are classified as category X (the risk of using these agents considerably outweighs any benefits) according to the most recent ESC 2018 guidelines [371]. However, it should be strongly emphasised that teratogenicity or the occurrence of congenital defects following statin exposure were mainly observed in experimental studies. Recent data do not confirm these findings. A recent systematic review and a meta-analysis of nearly 2.5 million cases [372] demonstrated no significant increase in congenital malformations after statin therapy. The authors emphasised that there was no evidence of teratogenic effects of statins during pregnancy, and this issue required further investigation, especially as more and more pregnant women were at high cardiovascular risk (women with established cardiovascular disease, women with HoFH) and might benefit from statin therapy [372]. In this context, the available data from South Africa, where statins were used in pregnant women with homozygous FH, also did not reveal any risks for either the mother or the child [373]. Recent studies and data also indicated the possibility of using pravastatin in pregnant women during the last trimester of pregnancy to prevent pre-eclampsia [370]. Unfortunately, the latest results of a randomised study involving 1120 patients did not confirm this relationship; however, once again, the safety of statins in this group of women has been confirmed [374].
It should be emphasised that in women receiving chronic statin therapy, the risk of harm to the foetus is not high, and in the case of accidental pregnancy, the woman should be reassured, and the gynaecologist-obstetrician should be immediately informed of the fact [8, 9]. The only safe lipid-lowering agents in pregnancy are ion exchange resins (currently unavailable in Poland) [8]. The best tolerated resin is colesevelam. In women with HoFH, continuation of LDL-apheresis during pregnancy is safe and indicated [8, 9]. According to the latest guidelines, it is also possible to consider ezetimibe and fenofibrate (when potential benefits outweigh the risk) [371].
Recommended methods of contraception in women of childbearing potential with FH include low oestrogen oral contraceptives, intrauterine devices, and condoms. Oral contraceptives with high oestrogen content may increase triglyceride and LDL-C concentration and therefore it is important to monitor lipid profile in women with FH using these agents. Medical consultations are also necessary for all women of childbearing potential whose parents have been diagnosed with FH, as the risk of homozygous FH in their offspring is as high as 25% [8, 9].
KEY POINTS TO REMEMBER
Lipid-lowering therapy should be discontinued in women at least 3 months before planned pregnancy, as well as during pregnancy and lactation.
Statins are not recommended due to the risk of teratogenicity, despite the lack of clear evidence confirming such a relationship.
More and more reports confirm the lack of risk of statins and the benefits of their use, especially in pregnant women with an underlying disease that threatens the life of the mother and the foetus (diagnosed cardiovascular disease or homozygous familial hypercholesterolaemia).
10.13. Cognitive disorders
Cognitive disorders comprise a heterogeneous group of conditions with respect not only to aetiology but also the extent of impairment, including mild memory disorders, mild cognitive impairment (MCID), and, in the most advanced form, dementia. Many systems are used for classification of dementia, including those based on the location of the causative brain damage, the type of symptoms, or the aetiopathogenesis. The most common cause of dementia are neurodegenerative diseases of the central nervous system (CNS), including Alzheimer’s disease (resulting from deposition of β-amyloid in the extracellular space of the central nervous system), accounting for ca. 50–70% of cases [375]. Dementia due to vascular disease is the second most represented group of diseases and accounts for approximately 15% of cases.
From the perspective of years and the available study results, it seems that lowering LDL-C concentration, and regardless of that the use of statins, reduces the amount of insoluble precursor protein for amyloid and has anti-inflammatory effects, which has a beneficial effect in terms of reduction of Alzheimer type neurodegenerative lesions. Reduction in LDL concentration is an established vascular protection factor. This has been confirmed in meta-analyses of tens of clinical trials concerning statins published in the last few years which demonstrated a significant reduction in Alzheimer’s dementia, vascular dementia, and generally mild cognitive impairment with statin use [376, 377], of which pleiotropic properties of this class of agents are supposed to be the cause.
Data concerning the effect of the method and rate of achieving the LDL treatment goal on neurocognitive functions depending on the age of patients receiving a specific therapy are still scarce. Data are available that suggest that lipid-lowering treatment reducing LDL concentration prevents development of cognitive deficits in middle-aged and elderly individuals, but not after the age of 80. In these patients, high concentration of low-density lipoproteins is not considered a risk factor for dementia (this may also be associated with the lipid paradox observed in this group of patients) [378, 379].
Another important issue is neurological safety related to LDL reduction. With this respect, evidence is available from studies with PCSK9 inhibitors, including a subanalysis dedicated to neuropsychological evaluation (the EBBINGHAUS study) [176, 184, 380]. In those trials, patients achieving LDL concentration below 30 mg/dl did not show any deterioration of their cognitive processes in comparison with those with higher LDL concentration. These results confirm the few previous observations in individuals with loss of function PCSK9 gene mutations who, despite extremely low LDL concentration, often below 30 mg/dl, showed no neurocognitive disorders [381]. This also proves slightly different mechanisms of lipoprotein circulation in the CNS and the impermeability of both the blood-brain barrier and the blood-cerebrospinal fluid barrier to cholesterol and plasma lipoproteins (except for the precursor of small spherical HDL particles). There are also single reports that a disorder of local (rather than plasma) lipoprotein metabolism in the central nervous system and cerebrospinal fluid is most likely the cause of decreased supply of cholesterol necessary for the recovery of myelin sheaths, which is probably associated with neurodegenerative diseases [382].
For certain, the results of many studies with statins have proven no deterioration of cognitive function in people receiving this treatment. Therefore, the ESC position on the impact of these products on cognitive functions remains neutral [9].
KEY POINTS TO REMEMBER
Treatment of lipid disorders in patients with neurocognitive disorders requires routine management based on the assessment of cardiovascular risk, determining the choice of therapy with adequate lipid-lowering potency.
There is no convincing evidence of increased risk of cognitive disorders as a result of the use of statins or in individuals with low LDL-C concentration.
On the contrary, scientific evidence supporting the protective effect of statins on the development of cognitive impairment, especially of vascular aetiology, is increasing.
10.14. Liver diseases
For years, increased aminotransferase activity was considered by physicians a contraindication to statins; as a result, patients with high cardiovascular risk often received no lipid-lowering therapy at all. Unfortunately, this is still the most common cause of statin dose reduction or treatment discontinuation [8, 152]. However, further experimental, and clinical trials as well as cohort studies have shown that in fact direct mechanisms that could contribute to hepatocyte damage in the course of statin therapy are still not fully known, and the phenomenon of asymptomatic elevation of aminotransferase activity in the course of treatment is rare (< 1%) and transient [8, 153]. In February 2012, the FDA was the first agency to recommend that liver enzyme (alanine aminotransferase – ALT) activity should be measured prior to initiation of therapy, and no routine monitoring is necessary during its continuation unless clinical symptoms develop. Further associations and expert opinions, including the International Lipid Expert Panel (ILEP) or the latest ESC/EAS 2016 guidelines, have maintained this opinion [8, 153], although they point to the possibility of assessing aminotransferase activity 8–12 weeks after treatment initiation or dose increase, which refers to treatment optimisation every 4–6 weeks [9].
Furthermore, available studies indicate that statin therapy should be continued, and patients may benefit even in case of chronic B (hepatitis B virus, HBV) and C hepatitis (HCV), although not those with acute or active forms, which is the only contraindication to statins [8, 153]. Among the benefits, there is a significant risk reduction of hepatocellular carcinoma (HCC) by up to 28% in patients with HBV and HCV, and reduction of the presence of hepatitis C virus in the blood by inhibition of its replication [8, 153]. Studies are also available that indicate the beneficial role of statins in patients with primary biliary cirrhosis (PBC), in terms of the effect on the course of the disease itself as well as the reduction of cardiovascular risk in this group of patients [153]. Even greater benefits may be observed in patients with non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), diseases which at present are much more common causes of cirrhosis than alcoholism [8, 153]. Hypertriglyceridaemia with decreased HDL-C concentration is common in the course of these diseases, as well as increased occurrence of atherogenic lipoprotein subfractions, i.e., VLDL and LDL [8, 153]. Available studies have demonstrated that statin therapy very effectively reduces the risk of cardiovascular morbidity and mortality in the group of patients with NAFLD in comparison with patients not receiving statins (68 vs. 39%, p = 0.007) [383]. Available studies also indicate that statin treatment in these patient groups is safe [9]. Therefore, in patients with NAFLD or NASH and baseline ALT > 3× the upper limit of normal (ULN), low-dose statins may be considered, with the need to monitor ALT monthly for 3 months and subsequently 4× per year [8, 9].
This has been confirmed by the results of the most recent meta-analysis of 9 studies evaluating the potential protective role of statins in patients with chronic viral liver disease [384]. The results demonstrated no significant difference in the risk of death from any cause between patients receiving and not receiving statins in the overall analysis. However, the risk of death was significantly reduced by 39% in patients receiving statins and followed-up for more than 3 years. In addition, the risk of HCC, fibrosis, and cirrhosis in statin users was reduced by 53%, 45%, and 41%, respectively. Interestingly, ALT and AST activity decreased slightly (and not increased!) after statin therapy; this reduction was not statistically significant [384].
KEY POINTS TO REMEMBER
Liver enzyme (ALT) activity should be measured prior to initiation of therapy (it may be considered during dose titration) and no routine monitoring is necessary during treatment continuation (unless clinical symptoms develop).
Due to the benefits related to the course of the disease itself and its complications, as well as reduced cardiovascular risk, statin therapy is recommended in patients with chronic hepatitis B and C.
In patients with NAFLD/NASH, statin therapy is safe, contributes to improved disease course, and significantly reduces cardiovascular risk.
The only contraindication to statin therapy is acute, active liver disease.
In patients with liver diseases, lipid disorders should be treated in consultation with a hepatologist/gastroenterologist.
10.15. HIV/AIDS
In terminal diseases and palliative conditions, careful assessment of the benefits and potential risk of adverse reactions in treatment of dyslipidaemia should be performed [385]. These groups of patients were typically excluded from large randomised clinical trials; therefore, the evidence is weak and leads to controversies and differences in the approach in guidelines published to date. Some studies also indicate that in palliative patients discontinuation of statin therapy was not associated with deterioration of cardiovascular parameters, including mortality, while significantly improving the quality of life of these patients [386, 387]. These data are still not sufficient to draw any conclusions; certainly, an individual approach to the patient should sometimes be considered, but one should always bear in mind that discontinuation of statin therapy may be associated with increased risk of cardiovascular events [153].
Patients with HIV/AIDS are such a difficult group of patients, with very scarce data from the studies. In this group, not only lipid-lowering therapy is important (in these patients, lipid disorders may occur as often as in general population), but particular attention should be paid to possible drug interactions, especially as these patients often receive multiple concomitant medications. Particular attention should be paid to interactions between statins and protease inhibitors in HIV patients due to metabolism via CYP3A4, leading to an increased risk of myopathy and rhabdomyolysis [9]. While in these patient groups TG and LDL-C concentrations are often decreased, treatment may negatively affect the lipid profile. Highly active antiretroviral therapy (HAART), primarily protease inhibitors, negatively affects the lipid profile, increasing in particular the risk of atherogenic dyslipidaemia [388]. If such lipid disorders are identified, the use of different agents in HAART may be considered; pravastatin may also be considered as it is recommended in patients with HIV due to its minimal metabolism by the cytochrome P450 isoenzyme system [8, 9]. The results of a recent study indicate that pitavastatin (available already in Poland), the metabolism of which practically does not involve cytochrome P450 isoenzymes (a few percent involvement of CYP 2C8 and 2C9), is more likely than pravastatin to contribute to a decrease in immune activation and arterial inflammation in HIV-infected individuals [389]. Furthermore, a subsequent study demonstrated that pitavastatin was more effective in reducing LDL cholesterol in this group of patients, with a safety profile comparable to that of pravastatin [390]. In addition to pravastatin and pitavastatin, other statins may be considered in treatment of dyslipidaemia in this group of patients, although dose adjustment may be necessary [391]. Detailed information on drug interactions in patients with HIV can be found at: www.hiv-druginteractions.org.
It is also worth noting that cardiovascular risk in a HIV patient is higher than in a patient without HIV (by up to 60% and more), and antiretroviral agents, in particular protease inhibitors, increase the risk as much as two-fold [392, 393].
KEY POINTS TO REMEMBER
In patients with HIV/AIDS, treatment should be selected depending on cardiovascular risk and the benefits the patient may obtain from long-term therapy.
In most HIV patients receiving antiretroviral therapy, non-pharmacological management is insufficient, and the addition of a statin should be considered. Pitavastatin and pravastatin are the preferred statins in this group. In case of statin intolerance, ezetimibe (or combination therapy in partial intolerance) is a treatment option.
10.16. Terminal diseases and palliative conditions
The aim of treatment of lipid disorders is to reduce cardiovascular events and mortality, as well as overall mortality. However, there is no evidence from clinical trials for the absolute benefit of statins in patients with terminal diseases and palliative conditions. For obvious reasons, such patients were excluded from randomised clinical trials.
A randomised clinical trial was conducted several years ago comparing the 60-day mortality in patients with an estimated life expectancy from 1 month to 1 year who decided not to receive statins with those who continued treatment [394]. The duration of previous statin therapy, in primary or secondary prevention, was at least 3 months. There were 189 patients in the treatment discontinuation group and 192 in the continuation group. The mean age of patients was 74.1 ±11.6 years. Of these, 48.8% suffered from cancer, and 22% had cognitive impairment. Mortality did not differ significantly between the treatment continuation group and those who discontinued therapy (23.8% vs. 20.3%; p = 0.36). The quality of life (QoL) was also assessed using the McGill questionnaire, and the occurrence of various complaints using the Edmonton Symptoms Assessment scale. It turned out that the quality of life of patients who discontinued statin therapy was significantly higher that of those receiving a statin (McGill score: 7.11 vs. 6.85; p = 0.04). Based on those results, the authors concluded that discontinuation of therapy in this group of patients is safe and beneficial due to improved quality of life [394].
What is the real-life approach to statin therapy in patients with limited life expectancy? A study conducted in New Zealand may serve as an example [395]. The rate of statin discontinuation in the last 12 months of life was evaluated in 20,482 individuals over the age of 75, including 4832 people with cancer. The treatment was discontinued in 70.4% of patients with cancer diagnosis and in 55% without this disease (p < 0.05), even in secondary prevention [395]. In recent joint guidelines of twelve American scientific societies on cholesterol management, the experts have stated that it is reasonable for people over 75 years of age to stop treatment if there is functional decline (physical or cognitive), multimorbidity, frailty, or reduced life expectancy [251]. In contrast, in the 2019 ESC/EAS guidelines the experts did not refer to statin therapy in patients with terminal diseases and palliative conditions [9].
Recently, a group of investigators reviewed 18 current guidelines on cardiovascular disease prevention with regard to recommendations on discontinuation of statin therapy in older adults [396]. In conclusion, they stated that “Current international CVD prevention guidelines provide little specific guidance for physicians who are considering statin discontinuation in older adults in the context of declining health status and short life expectancy”, indicating that this topic is often overlooked in the guidelines on prevention and treatment of cardiovascular diseases [396].
Therefore, the decision is difficult and should apparently be made on an individual basis. Continuation of statin therapy in terminal patients and in palliative conditions does not prolong life, and apparently impairs its quality! On the other hand, in patients at high cardiovascular risk receiving statin treatment for a long time without adverse reactions, continuation of therapy may be considered in order to avoid a possible cardiovascular event.
Finally, the opinion from an article by Prof. Banach and Dr. Serban may be cited: “(…) it needs to be emphasised that the available data are not sufficient to draw any direct conclusions or recommendations, and any reduction in the statin dose or discontinuation should be balanced with the increased risk of cardiovascular events” [385].
KEY POINTS TO REMEMBER
In terminally ill patients and those receiving palliative treatment, data indicate that discontinuation of statin therapy does not increase the risk of cardiovascular events and may improve their quality of life.
Therefore, in these patients decisions should be made on an individual basis, taking into consideration the patient’s life expectancy and the potential to reduce cardiovascular risk with statin therapy, the occurrence of adverse effects and drug interactions with statins and, which should be particularly emphasised, the patient’s quality of life.
10.17. Viral diseases including COVID-19
The coronavirus pandemic laid bare the shortcomings of the Polish healthcare system, showed very weak patient education on health and, consequently, contributed to significant deterioration of population health in every aspect, particularly in the context of cardiovascular diseases.
Observations to date point to a number of factors associated with worse course of SARS-CoV-2 infection [397]. The most commonly reported factors include diabetes and obesity [398, 399]. The possibility of cardiovascular events in the course of COVID-19, including myocarditis, acute coronary syndrome, or thrombotic complications, is also emphasised. Despite concerns expressed at the beginning of the pandemic, no adverse relationship between the use of the renin-angiotensin system inhibitors and the risk of development and the course of COVID-19 has been proven [400, 401]. It should be emphasised that certain previous observations indicate that the renin-angiotensin system inhibitors and statins may reduce the risk of death due to pneumonia [400]. Study results also indicate at least neutral effect of statins on the risk of development and the course of COVID-19. In contrast, the number of studies indicating their very important role, improving the prognosis not only in the course of COVID-19, but also after recovery, in the so-called Long-Covid period (> 12 weeks after recovery), is increasing [402]. This is associated with the mechanisms of action of statins, not only their anti-inflammatory and anti-oxidative properties, stabilising atherosclerotic plaque (especially during the so-called cytokine storm), but also inhibition of the main coronavirus protease, reduction of the availability of lipid structural components of the virus envelope, degradation of so-called viral lipid rafts, or inhibition of its replication [403–405]. Some observations indicate potential benefits of statins (used prior to hospitalisation) on the course of COVID-19, manifested by reduced risk of severe course and death [406, 407]. One of the recent meta-analyses of 24 studies including over 32,000 patients has demonstrated that statin use significantly reduced the risk of admission to the intensive care unit in the course of COVID-19 (by 22%) and mortality (by 30%), with no significant effect on the risk of intubation. An additional analysis showed also that the risk of death was even lower if statins were used in hospital settings in patients with COVID-19 (60% risk reduction, 95% CI: 0.22–0.73) in comparison with prehospital use alone (23% reduction) [408].
In patients with COVID-19, due to possible use of antiviral, antiretroviral, or antirheumatic agents, consideration should be given to the possibility of drug interactions with statins and statin intolerance. In this case, the ILEP 2020 recommendations should be followed, in which possible interactions have been discussed in detail in the guidelines for patients with FH [157].
Regarding management of lipid disorders during the COVID-19 pandemic, the following recommendations should be proposed, presented in detail in Table XXXVII.
Table XXXVII
Recommendations on treatment of lipid disorders in patients with COVID-19
11. Adverse effects associated with treatment of dyslipidaemia/statin intolerance
Statin intolerance is a phenomenon that has been observed for years, but the interest in it in recent years is associated with the introduction of new agents in combination therapy (PCSK9 inhibitors, inclisiran, and bempedoic acid) (Section 9.10). Non-adherence is associated with intolerance, as adverse reactions associated with statin use are the most common cause of non-adherence or treatment discontinuation. To this, reluctance to use statins and the effect of drucebo (the term introduced by Prof. Banach in the ILEP [409, 410]), i.e., adverse reactions observed in patients receiving a specific agent, but not being a result of its use, which may account for > 70% of all post-statin symptoms, should be added [152, 153, 410].
According to the results of the most recent meta-analysis, including data from more than 4 million patients, the global incidence of statin intolerance is 9.1%, and if intolerance is diagnosed using existing definitions, including the ILEP definition [153], the incidence ranges from 5.9% to 7% [411]. Statin intolerance should be defined as inability to receive statin therapy adequate (with respect to the product or the dose) to the existing cardiovascular risk [8]. In other words, statin intolerance is not only the lack of statin treatment due to clinical or biochemical symptoms (so-called complete intolerance, which affects only 3–5% of patients), but also the phenomenon of underdosage or the use of a statin too weak in relation to the cardiovascular risk [8]. In March 2015, the International Lipid Expert Panel (ILEP) proposed a new definition of statin intolerance [153] (Table XXXVIII).
Table XXXVIII
Definition of statin intolerance proposed in the ILEP recommendations (2015)
Therefore, one of the most difficult challenges is not only the right management, but above all the right, objective diagnosis of true statin intolerance. In this context, the authors of these guidelines recommend the use of the Statin-Associated Muscle Symptom Clinical Index (SAMS-CI) for objective assessment whether reported muscle pains are associated with statin treatment [412] (Table XXXIX).
Table XXXIX
Modified Statin-Associated Muscle Symptom Clinical Index (SAMS-CI) [412]
| SAMS-CI | Score |
|---|---|
| 1. Location and pattern of muscle symptoms (if more than one category applies, record the highest number) | |
| Symmetric, hip flexors or thighs | 3 |
| Symmetric, calves | 2 |
| Symmetrical, proximal upper extremity* | 2 |
| Asymmetric, intermittent, or not specific to any area. | 1 |
| 2. Timing of muscle symptom onset in relation to starting statin regimen | |
| < 4 weeks | 3 |
| 4–12 weeks | 2 |
| > 12 weeks | 1 |
| 3. Dechallenge – timing of muscle symptom improvement after withdrawal of statin | |
| < 2 weeks | 2 |
| 2–4 weeks | 1 |
| No improvement after 4 weeks | 0 |
| 4. Rechallenge – timing of recurrence of similar muscle symptoms in relation to starting second regimen | |
| < 4 weeks | 3 |
| 4–12 weeks | 1 |
| > 12 weeks or similar symptoms did not reoccur. | 0 |
| INTERPRETATION: (likelihood that the patient’s muscle symptoms are due to statin use): | Probable 9–11 Possible 7–8 Unlikely 2–6 |
It should be noted that there are many risk factors which may increase the chance for statin intolerance, including but not limited to: physical activity, especially after initiation or increase in intensity; liver and/or kidney disease, hypothyroidism, vitamin D deficiency [413], alcohol consumption, rheumatic diseases, major surgical procedures, low body weight, female gender, or elderly age [8, 153]. These risk factors were most cited as expert opinions and have never been confirmed with respect to potential causality or simply association with development of statin intolerance. In the meta-analysis mentioned above [411], the first attempt at such validation has been made. The most important risk factors for intolerance were: elderly age (OR = 1.33; as a continuous variable), female gender (1.48), Asian (1.25) or African origin (1.29), diabetes (1.27), obesity (1.31), hypothyroidism (1.38), chronic liver (1.24) or kidney disease (1.25), alcohol consumption (1.22), exercise (1.23), the use of antiarrhythmic agents (1.31), calcium channel blockers (1.36) or statins, primarily at high doses (1.38) [411].
Discussing the phenomenon of intolerance, attention should be paid to several key elements. Symptoms of intolerance in 90% occur within the first 6 months after initiation of statin therapy or dose increase, and in 75% within the first 12 weeks of this therapy [414]. Intolerance symptoms are unlikely to occur 1 year after treatment initiation or dose increase, unless a factor increasing this risk appears (disease exacerbation, a new medication interacting with statins) [414]. The most common reasons of statin intolerance are muscle symptoms manifested as pain (myalgia), muscle cramps or weakness, with or without elevated creatine kinase (CK) activity (myopathy), with or without inflammation (myositis) [415]. Myonecrosis and rhabdomyolysis are extremely rare (< 2/100,000 patient-years) and may often be associated with genetic predisposition, exacerbation of a concomitant disease, or treatment error [156, 414]. Other symptoms, with a confirmed causal relationship to statin administration, include new cases of diabetes and temporary elevation of alanine aminotransferase activity [415]. Other symptoms of statin intolerance mentioned in the literature, including hair loss, sleep disturbances, flu-like symptoms, lupus-like symptoms, rashes, gastrointestinal symptoms, decreased libido, and gynaecomastia, are very rare and their causal relationship to statin use has not been confirmed [153, 156, 415].
In statin-intolerant patients, the appropriate management (so-called step-by-step approach, i.e., thorough history taking and gradual exclusion of reasons for intolerance, prompt initiation of appropriate management) may contribute to the fact that more than 95% of those patients may still receive statins [416]. Currently, in the management of patients with statin intolerance, the dominant rule is to try to retain even the lowest statin dose that is tolerated and/or use it even every 2–3 days (data suggest this possibility for atorvastatin and rosuvastatin [307]), and in the case of complete statin intolerance, after discontinuation, especially in high-risk patients, ezetimibe [109] and other non-statin therapies should be introduced immediately (bempedoic acid, which in this year will be available in Poland, PCSK9 inhibitors, inclisiran, and nutraceuticals or their combinations with proven lipid-lowering effect [136]). It is also worth noting that pitavastatin is already available on the market, which, due to its metabolism (practically no involvement of CYP450) and properties (bioavailability 50%) has potentially the lowest risk of intolerance in the form of myalgia (estimated at ca. 2% for 4 mg) or new cases of diabetes (estimated at ca. 4.5% for the highest dose); in both cases, these values are comparable with those for placebo. Detailed guidelines for management of statin intolerance are presented in Figures 8 and 12, and Table XVII.
12. Recommendations on monitoring lipids and biochemical parameters during treatment of lipid disorders
In this section, recommendations presented in the ILEP 2015 position [153] and EAS 2015 [417] as well as European guidelines (ESC/EAS) on the management of dyslipidaemia (2019) are summarised and approved [9]. Statins are the most commonly used agents reducing LDL-C concentration; therefore, most attention was paid to their safety. The most common adverse effects associated with statin therapy are muscle symptoms (SAMS), usually pain (myalgia), muscle weakness, and cramps. One of the most serious muscle symptoms is myopathy, particularly rhabdomyolysis, which requires immediate hospitalisation. The manifestations of rhabdomyolysis include marked elevation of creatine kinase (CK) activity, increased myoglobin concentration with myoglobinuria (dark urine), and acute renal failure with increased creatinine and potassium concentration [8, 9].
According to the ESC/EAS (2019) experts, prior to initiation of pharmacotherapy lipid parameters should be assessed at least twice (except for patients with ACS) at intervals of 1–12 weeks, and after 6 ±4 weeks following treatment initiation. Lipid concentration should also be assessed after 6 ±4 weeks following the change of lipid-lowering therapy, until the target LDL-C concentration has been achieved [9]. Then lipids should be tested at 6–12-month intervals. At the same time as the baseline lipid profile, CK and alanine aminotransferase (ALT) activity should be assessed, and HbA1c or glucose concentration measurement should be considered. The last two tests and their monitoring are applicable to patients at high risk of diabetes mellitus, those on high-dose statin therapy, the elderly, obese individuals, and those with metabolic syndrome. This requirement is associated with potential diabetogenic effect of statins.
Statin therapy is not initiated if ALT ≥ 3× upper limit of normal (ULN) or CK ≥ 4× ULN [9]. Routine monitoring of these enzymes is unnecessary during statin therapy, although European experts recommend an ALT measurement 8–12 weeks after treatment initiation and after dose increase, and then only in case of alarming symptoms [9]. Experts also remind that mild transient increase in ALT activity may occur during treatment with statins, which disappears with continued treatment (Section 10.14). An indication for ALT activity measurement is development of liver symptoms during treatment (pain, weakness, jaundice), and development of muscle symptoms for CK measurement.
The situation is different during treatment with a fibrate; in this case, ALT activity should be monitored regularly, and prior to introduction of this agent, creatinine should be measured, in addition to ALT and CK.
Continuation or cessation of pharmacotherapy depends on whether ALT < 3× ULN or ≥ 3× ULN. If ALT < 3× ULN, treatment can be continued and the test repeated after 4–6 weeks (usually, the activity normalises in this period); if ALT ≥ 3× ULN, treatment should be interrupted or the dose reduced (which is preferred by the authors of these guidelines), the test repeated after 4 weeks, and the therapy gradually resumed after normalisation of ALT activity.
The indication for CK assessment is development of muscle symptoms, which may be accompanied by a CK activity increase of varying degrees. Occasionally, increased CK activity is detected in a patient without muscle symptoms. A decision on whether to continue or discontinue treatment is based on the presence or absence of SAMS and the increase in CK, i.e. < 4× ULN or ≥ 4× ULN [9] (Figure 12).
Statin therapy may be continued, if:
CK < 4× ULN in a patient without muscle symptoms (the patient should be informed of the possibility of symptoms and CK activity should be measured).
CK < 4× ULN and muscle symptoms:
CPK ≥ 4 × ULN but < 10× ULN without muscle symptoms:
Statin therapy should be discontinued immediately, if:
CK > 10× ULN:
CPK ≥ 4× ULN but < 10× ULN with muscle symptoms:
CK < 4× ULN and persistent muscle symptoms making it impossible to function:
CK within normal values but muscle symptoms intolerable,
In statin-intolerant patients, the following treatment options should be considered when CK activity returns to normal: dose reduction of the same statin, use of another statin, statin administration every other day or once/twice a week, combination pharmacotherapy (including new agents), and lipid-lowering nutraceuticals [415].
KEY POINTS TO REMEMBER
Prior to initiation of pharmacotherapy, lipid concentration should be assessed at least twice.
The first lipid assessment is performed after 6 ±4 weeks following the initiation of pharmacotherapy. This interval should also be maintained after treatment modification.
If treatment goals are achieved, it is sufficient to control lipid concentration once or twice per year.
Muscle symptoms comprise the most common adverse effect of statins. Hepatotoxicity is rare and transient.
Prior to statin treatment, ALT and CK activity should be measured, and HbA1c or glucose measurement should be considered; ALT and CK activity and creatinine concentration should be assessed prior to fibrate treatment.
Treatment should not be initiated if CK ≥ 4× ULN or ALT ≥ 3× ULN.
There is no need for routine assessment of CK and ALT. The test is performed when muscle or hepatic symptoms develop.
13. Causes of ineffective treatment of lipid disorders
Effective treatment of lipid disorders means, in the first place, achievement of the target LDL cholesterol concentration. However, successful treatment does not translate into the patient’s well-being, but into the number of cardiovascular events avoided. The key to successful treatment of dyslipidaemia is to create the right relationship between the physician and the patient, which allows the patient to fully understand the purpose and expected effects of treatment.
13.1. Efficacy of treatment of dyslipidaemia
The term “compliance” is very broad and difficult to define precisely; formally, it is defined as “the act or process of doing what one has been asked or ordered to do”. In medical context, this refers to medical advice. Putting it simply, compliance can be determined on the basis of the efficacy of treatment of a specific condition, in this case dyslipidaemia. This efficacy has improved over recent years. According to the results of the 3ST-POL study, which was conducted in the years 2007–2008, the recommended total cholesterol concentration was achieved in less than 10% of patients with and less than 16% of patients without cardiovascular risk, and the target LDL concentration in 15.6% and 22.7%, respectively. However, that study involved only outpatients [418]. In the same period, according to the Polish results of the EUROASPIRE study, the target total cholesterol concentration was achieved in up to 70% of patients after myocardial infarction and the target LDL-C in 39% of patients [419]. The latest data on the efficacy of treatment of hypercholesterolaemia have been provided by the WOBASZ II study (2013–2014). With regard to the general population of Poland, only 6% of patients with hypercholesterolaemia are treated effectively, 15% are treated ineffectively, and others either are not aware of the disease or receive no pharmacological treatment. In comparison with the WOBASZ study in the years 2003–2005, a significant increase in the percentage of patients receiving pharmacotherapy as well as the percentage of patients treated effectively was observed [21].
13.2. Therapeutic persistence in treatment of dyslipidaemia
Studies demonstrate that a large proportion of patients quickly discontinue treatment of hypercholesterolaemia. According to the 3ST-POL study [418], 25% of patients discontinued statins as early as 3 months after treatment initiation. After 3 years, only 15% of patients were using statins. An analysis of data from the National Health Fund demonstrated that therapeutic persistence with statins in Poland is definitely too low [420]. Only 12% of patients met the current criteria for adherence and therapeutic persistence. In addition, the results of a pilot analysis of almost 200,000 e-prescriptions issued in 2018 in Poland indicated that as much as 20.8% of them were not filled. For statins, this percentage was 17.5% (simvastatin 14.3%; atorvastatin 18.9%; rosuvastatin 17.4%) [421]. It should be emphasised that this applies only to single prescriptions, which may translate into a significant proportion of patients who discontinue or periodically interrupt long-term treatment.
An analysis of adherence in patients post myocardial infarction or stroke suggests high variability of therapeutic persistence over time, both in terms of improvement and worsening of adherence [422]. This indicates that adherence must be closely monitored, especially in patients with a history of a cardiac or cerebrovascular event.
Treatment discontinuation is a very serious problem and may be a result of the decision of either the patient or the physician. This is extremely dangerous because, particularly in high-risk and very high-risk individuals, it may be associated with plaque instability and the risk of a (recurrent) cardiovascular event [8, 9]. It has been demonstrated that strict adherence (≥ 90%) to statin therapy in comparison with < 50% adherence (evaluated by means of the medicine possession ratio) is associated with a 30% increase in the risk of death in a nearly 3-year follow-up [423].
13.3. Therapeutic inertia
The role of a physician in the therapeutic process is clearly essential. In treatment of dyslipidaemia, the physician is the person who assesses the overall cardiovascular risk, confirms indications for the initiation of pharmacotherapy, decides on the choice of a specific agent or agents, as well as monitors the safety and efficacy of the selected treatment regimen. In Poland, especially in outpatient care, a problem of so-called therapeutic inertia has been observed for years. In the context of treatment of dyslipidaemia, the following issues are primarily observed:
Underestimation of the importance of non-pharmacological interventions;
Unnecessary deferral of the introduction of pharmacotherapy of dyslipidaemia;
The use of too low statin doses in relation to the intended treatment goals;
Treatment failure not resulting in modification of statin dose;
Unjustified discontinuation of statin therapy;
Fear of using high-dose statins or combination therapy;
Failure to perform tests to monitor treatment efficacy.
Analysis of the WOBASZ and WOBASZ II studies made it possible to analyse trends in high-dose statin treatment over a decade. At all levels of cardiovascular risk, an increase in the proportion of patients receiving high-intensity or moderate-intensity statin treatment, as well as a reduction in the proportion of those receiving low-dose statins and the proportion of individuals treated with diet alone was observed. Moreover, the most noticeable change occurred in patients with a very high cardiovascular risk, of whom 18% were receiving high-dose therapy, another 49% of patients were receiving moderate-intensity statin treatment, and only 10% were treated with diet alone [419]. The use of statins in higher and moderate doses was associated with higher proportions of patients achieving their treatment goals (30% and 33%, respectively) as compared to 25% of patients using low-dose statins. It should be emphasised that despite the observed improvement, still too few patients receive high-dose statins, i.e. only 17.9% and 7.7% of patients with very high and high cardiovascular risk [419].
13.4. Causes of ineffective treatment of lipid disorders
When considering the causes of ineffective treatment, a number of demographic and socioeconomic factors should be taken into consideration. In most studies, it was demonstrated that women were less compliant with statins than men [424]. Age is another factor associated with statin adherence. It is worth emphasising that the relationship between adherence to statins and age has the shape of reverse U curve, i.e., the worst adherence is observed in young patients (< 50 years) and the elderly (≥ 70 years) [425]. Race has also been shown to affect the adherence to statins. African Americans are significantly less compliant. The income and education level also affect the compliance rate, i.e., lower-income patients are less likely to adhere to statin treatment than those with higher income. Similarly, individuals with a low level of education are less compliant than those with higher education [426]. Concomitant diseases may affect adherence in different ways. Depression, dementia, malignancies, renal and pulmonary diseases, including obstructive sleep apnoea, are associated with poorer adherence to statin therapy [426]. On the other hand, diabetes mellitus, obesity, or a history of stroke or myocardial infarction are associated with better compliance [426]. In addition, Lemstra et al. demonstrated that patients prescribed with statins for the first time followed the recommendations worse than those previously receiving a statin [427]. All the factors discussed above can be classified as non-modifiable factors affecting adherence to lipid-lowering therapy.
Niklas et al. conducted an analysis of factors associated with achievement of the treatment goal in hypercholesterolaemia in relation to the population of Poland as a part of the WOBASZ II study [300]. Younger age, diabetes mellitus, non-smoking status, concomitant cardiovascular disease, and the number of physician visits ≥ 4/year have been shown to be independently associated with keeping cholesterol concentration within the target range.
Non-compliance with statin therapy is also affected by a number of modifiable factors (Figure 13) [428]. It is worth emphasising that they are often easy to establish and, most importantly, their discussion with patients and modification is possible. One of the most important issues is to present the disease to the patient and the effect of elevated cholesterol values on their health in a comprehensible way [428]. This knowledge may contribute to the elimination of one of the most significant reasons for non-compliance, i.e., questioning of the health risk associated with elevated cholesterol concentration, and therefore the justification of the therapeutic process. Therefore, a proper doctor-patient relationship should be a tool to improve treatment efficacy [425, 428]. Another very important aspect of improvement of treatment of lipid disorders is simplification of the therapeutic regimen, for example by reducing the number of tablets. It is postulated that the use of combination products (statin + ezetimibe or statin + antihypertensive agent/agents) may increase treatment efficacy. Furthermore, the available data indicate that this improvement in adherence may result in a better prognosis in comparison with treatment with the same agents at the same doses, but not in combination products [429].
13.5. Recommendations on how to improve doctor-patient cooperation and thus the efficacy of lipid-lowering therapy, and effectively fight against anti-statin movements
Statins have been shown to be effective in reducing cardiovascular events and mortality, and even though they are very affordable, they remain significantly unused. Widespread claims of adverse effects that are enhanced and promoted by the media have been consistently associated with adverse effects on the use of statins worldwide. While the academic community must strive to work together with the media to harmonise public health messaging, physicians caring for their patients play a key role in reducing disinformation and actively stopping its effect on the vicious cycle of the falsely attributed adverse effects and the effect of nocebo [430]. An analysis performed in the UK indicated that during a period of increased public discussion on the risk of statin use, a transient increase in the proportion of patients who discontinued statin therapy was observed. This study highlights the potential of widely discussed health-related messages and the impact of media on healthcare behaviour [431].
KEY POINTS TO REMEMBER
Effective treatment of lipid disorders is the achievement of the target LDL cholesterol concentration. The measure of treatment success is not the patient’s well-being but the number of cardiovascular events avoided. The key to successful treatment of dyslipidaemia is to create the right relationship between the physician and the patient, which allows the patient to understand in a proper way the purpose and expected effects of treatment;
The latest data from the WOBASZ II study indicate that with regard to the general population of Poland, only 6% of patients with hypercholesterolaemia are treated effectively, 15% are treated ineffectively, and others either are not aware of the disease or receive no pharmacological treatment; the results were slightly better in the Da Vinci study, in which only 17% of patients in Poland at very high cardiovascular risk achieved their treatment goals;
A number of modifiable and non-modifiable factors associated with statin non-adherence have been identified. It is worth emphasising that the modifiable factors are often easy to establish and, most importantly, their discussion with patients and modification is possible.
Adherence must be closely monitored, especially in patients with a history of a cardiac or cerebrovascular event;
The academic and scientific community must strive to work together with the media to harmonise public health messaging; physicians caring for their patients play a key role in reducing disinformation and actively stopping its effect on the vicious cycle of the falsely attributed post-statin adverse effects.
14. Organisation of healthcare in lipid disorders in Poland
The main burden of prevention as well as diagnostics and treatment of lipid disorders lies with family physicians (primary healthcare – PCH/primary care physicians – PCP) [432]. Their role consists in:
Early diagnosis of dyslipidaemia,
Determining the type of lipid disorders and establishing the diagnosis,
Patient and family education,
Treatment initiation and monitoring,
Determination of indications and referral for specialist consultations,
Cooperation with a specialist,
Detection of lipid disorders in family members,
Diagnosing complications and organ damage at an early stage.
In prophylactic and therapeutic management, lifestyle modification, including improper dietary habits, should always be taken into consideration; this may require cooperation with a dietician [13]. Treatment of lipid disorders requires cooperation between primary care physicians and specialist care physicians (internists, paediatricians, cardiologists, specialists in metabolic diseases, diabetologists, nephrologists, neurologists, or geriatricians).
Screening for dyslipidaemia should be performed in individuals with at least 1 risk factor for cardiovascular disease (e.g. arterial hypertension, obesity, tobacco dependence, positive family history) and in all men aged > 40 years, in women aged > 50 years, in postmenopausal women, in women with diabetes, in pregnant women, those with hypertension during pregnancy, in HIV-infected patients or those receiving HAART therapy, in men with erectile dysfunction, and in cases in which symptoms suggestive of cardiovascular diseases are present (Table XL).
Table XL
Recommendations on the assessment of lipid profile
In Table XLI the level of care at which a patient with dyslipidaemia should be treated is presented [433, 434]. Only good cooperation and continuous communication (e.g., organised as a part of coordinated care in primary prevention of cardiovascular diseases) between specific levels may guarantee appropriate and effective care for patients with lipid disorders.
Table XLI
Patient characteristics and levels of care in the healthcare system at which care is provided to patients with lipid disorders, including FH
While discussing the organisation of care for patients with lipid disorders in Poland, it seems necessary to mention the Prevention 40 PLUS programme, introduced by the Ministry of Health on July 1st, 2021, which constitutes a good beginning for coordinated care programmes in primary prevention. The programme has significant limitations in terms of the type and scope of tests, the lack of continuity of care (one-time package), and the lack of wide health-related education, which would be the best motivator to undergo such tests for young people, a majority of whom do not feel any disease; however, this is a step in the right direction (assuming widespread implementation of this programme) in order to make prevention of cardiovascular diseases and other chronic diseases in Poland real at long last. The scope of possible tests is presented in Table XLII. The authors of these guidelines encourage the dissemination of information on the programme, promotion of the programme among patients, and continuation of work with the Ministry of Health and the payer to extend this programme to a full-fledged programme of coordinated care for primary prevention of cardiovascular and chronic diseases in Poland.
Table XLII
Scope of tests that can be performed in the Prevention 40 PLUS programme