Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease characterized by persistent respiratory symptoms and airflow limitation, usually due to airway and alveolar abnormalities caused by exposure to harmful particles or gases [1]. Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is an important cause of poor prognosis for patients, as demonstrated by worsening respiratory symptoms and the need to change daily medications [2]. Currently, World Health Organization (WHO) statistics show that COPD is the third leading cause of death worldwide [3]. According to the latest epidemiological survey on COPD in China, the prevalence of COPD among people aged 40 years and older reached 13.7%, a significant increase from the 8.2% reported 10 years ago, and it is estimated that there are approximately 100 million cases of COPD in China [4, 5]. With the advent of a globally aging society, the morbidity and mortality rates of COPD will continue to rise, which will impose a huge economic burden on the world. Despite significant advances in the treatment of AECOPD, the prognosis for patients with severe AECOPD remains relatively poor. Patients with severe AECOPD require early assessment and intervention.
Compared to single variables, predictive scores that include multiple variables provide a better assessment of a patient’s condition. Predictive scores can provide strong support for risk stratification of patients and contribute to clinical management, including home treatment and early discharge of low-risk patients, as well as early identification and appropriate palliative care of high-risk patients. Some prediction scores have been available to predict in-hospital mortality in patients with AECOPD, such as DECAF [6], CRUB-65 [7], and BAP-65 [8]. The DECAF score, which includes five variables – dyspnea, eosinopenia, consolidation, acidemia and atrial fibrillation – has been shown in several studies to have a good predictive value for death during hospitalization in patients with AECOPD [9, 10]. Meanwhile, CURB-65 and BAP-65 have been used internationally to predict the prognosis of AECOPD and other respiratory diseases [11, 12]. A multicenter study established the ABCDMP score to predict in-hospital death in patients with AECOPD and cardiovascular disease and compared it with DECAF, CURB-65, CAP-65 [13]. However, depending on the level of medical care and lifestyle habits in local areas, there are significant differences in the in-hospital mortality rates of AECOPD patients [14]. The applicability of these scores in various populations needs more validation. In addition, patients with severe AECOPD tend to have more complex and progressive disease, making prognostic assessment more challenging. Therefore, new scores are needed to assess and predict in-hospital mortality in patients with severe AECOPD.
The purpose of this study is to develop and validate a predictive score that can simply and accurately predict in-hospital mortality in patients with severe AECOPD to help clinical practitioners assess the prognosis of these patients.
Material and methods
Study design and patients
This study consecutively included all patients older than 40 years who were admitted to Beijing Hospital for severe AECOPD from January 2011 to October 2022. AECOPD is considered to be exacerbated dyspnea with increased or purulent cough and/or sputum that requires additional care [2]. All diagnoses, namely, the primary and five secondary diagnoses, were made according to the International Classification of Diseases, Tenth Revision (ICD10) coding system. The patients in this study with severe AECOPD were patients admitted to the Respiratory Intensive Care Unit (RICU), and patients in the general respiratory ward who were diagnosed with respiratory failure or required mechanical ventilation during their hospitalization. The exclusion criteria for this study were hospitalization of less than 24 h, readmission within one month, and lack of partial medical information. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing hospital (BJ-2018-199).
In this study, a development cohort was created by randomly selecting 70% of patients from the overall population using statistical software, and the remaining 30% of patients were assigned to the validation cohort. We analyzed the development cohort to derive variables that had a significant effect on hospitalized patient mortality and assigned a score to each variable. The scores for all independent predictor variables were summed to produce a total mortality score for each patient. Finally, we assessed the predictive ability of the scoring system using the area under the receiver operating characteristic curve (AUROC).
Data collected
We collected clinical data on patients from the electronic medical record system, which consisted mainly of demographic characteristics and laboratory test results. Demographic characteristics included age, sex, body mass index (BMI), smoking status, length of stay, long-term home oxygenation, and confusion. Laboratory blood tests included red blood cell count, white blood cell count (WBC), platelet count, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), C-reactive protein (CRP), N-terminal pro-brain natriuretic peptide (NT-proBNP), D-dimer, creatinine, uric acid, fibrinogen and urea. Blood samples were collected from all patients within 24 h of admission, and the results of the first sampling were chosen.
The comorbidities we included were respiratory failure, coronary heart disease (CHD), chronic heart failure (CHF), atrial fibrillation, hypertension, diabetes, cerebrovascular disease, chronic kidney disease (CKD), gastroesophageal reflux (GER), anemia and sleep apnea hypopnea syndrome (SAHS).
Statistical analysis
The statistical software SPSS 26.0 was used throughout this study to analyze and process the data. All cases were described using frequencies and percentages for categorical variables and means and standard deviations for continuous variables. We used χ2 tests and t-tests to analyze these variables for comparison.
We performed univariate analysis in the development cohort and included variables with p < 0.10 in a multivariate logistic regression score to identify risk factors associated with short-term mortality in COPD. Factors with p < 0.05 in the multivariate analysis were ultimately selected for inclusion in the prediction score. The multivariate analysis demonstrated OR values and 95% confidence intervals. We assigned a value to each risk factor to develop a prediction score. The area under the receiver operator characteristic curve (AUROC) was used to evaluate the performance of the score in predicting hospital mortality. The goodness of fit was calculated by the Hosmer-Lemeshow statistic. At the same time, we reassigned the patients’ indicators according to the CURB-65, DECAF, m-DECAF, and BAP-65 risk scores and constructed their ROC curves and calculated the AUROC.
Results
Characteristics of all patients
A total of 488 patients aged 40 years or older who met the inclusion and exclusion criteria were included in this study. After we randomly assigned patients in a 7 : 3 ratio, 342 patients were assigned to the development cohort, and the remaining 146 were assigned to the validation cohort.
In the total sample, a total of 53 patients died during hospitalization, with a mortality rate of 10.9%. In-hospital mortality did not differ significantly between the development and validation cohorts (p = 0.364). The mean age of the total population was 78.0 ±8.2 years, and 361 (74.0%) patients were male. The most common comorbidities during hospitalization in patients with severe AECOPD were respiratory failure (77.5%), CHD (30.3%) and CHF (27.7%). There were no significant differences between the factors of patients in the development and validation cohorts, and the results of the analysis are shown in Table I.
Table I
Descriptive characteristics in development and validation cohorts
[i] Date are presented as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. BMI – body mass index, CHD – coronary heart disease, CHF – chronic heart failure, CKD – chronic kidney disease, GER – gastroesophageal reflux, SAHS – sleep apnea hypopnea syndrome.
Comparisons between survivors and non-survivors in the development cohort
The development cohort of this study ultimately included 342 patients, 40 of whom died during hospitalization. The results of the comparison of patient demographic characteristics and laboratory indicators between the non-survivor and survivor groups are shown in Table II.
Table II
Comparison of demographic and clinical characteristics of patients between non-survivors and survivors in development cohort
[i] Date are presented as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. BMI – body mass index, CHD – coronary heart disease, CHF – chronic heart failure, CKD – chronic kidney disease, GER – gastroesophageal reflux, SAHS – sleep apnea hypopnea syndrome, NLR – neutrophil/lymphocyte ratio, PLR – platelet/lymphocyte ratio, CRP – C-reactive protein, NT-proBNP – N-terminal pro-brain natriuretic peptide, PaO2 – arterial partial pressure of oxygen, PaCO2 – arterial partial pressure of carbon dioxide, PaO2/FiO2 – oxygenation index.
The mean age of patients in the development cohort was 78.2 ±8.2 years, and 76% of patients were male, but age and sex did not differ significantly between the two groups. Patients who died during hospitalization had longer lengths of stay (28.5 ±24.7 vs. 18.2 ±14.0 days, p = 0.013). More patients in the death group had altered confusion (65.0% vs. 32.4%, p < 0.001) during hospitalization. More patients who died in hospital had a combination of chronic heart failure (50.0% vs. 24.2%, p < 0.001) than the survivors.
In non-survivors, the white blood cell count was higher (10.3 ±6.5 vs. 8.1 ±3.4 × 109/l, p = 0.047), while the red blood cell (3.8 ±0.8 vs. 4.1 ±0.6 × 109/l, p = 0.039) and lymphocyte (0.8 ±0.6 vs. 1.1 ±0.6 × 109/l, p = 0.012) counts were both lower. The NLR was significantly higher (19.9 ±20.3) in patients in the death group than in those who survived (10.4 ±18.1, p = 0.007). In addition, patients in the death group also had lower albumin (33.2 ±6.0 vs. 35.4 ±5.7 g/l, p = 0.020) and PaCO2 (45.5 ±2.4 vs. 53.6 ±7.3 mm Hg, p = 0.005), while pH (7.39 ±0.05 vs. 7.37 ±0.07, p = 0.046) was higher than that in the survivor group.
Development and validation of the new prediction score
Table III presents the multivariate analysis of the statistically significant variables associated with in-hospital death in patients with severe AECOPD. The analysis showed that lymphocyte count < 0.8 × 109/l (OR = 3.26, 95% CI: 1.55–6.86, p = 0.002), age > 80 years (OR = 2.26, 95% CI: 1.07–4.77, p = 0.033), confusion (OR = 3.35, 95% CI: 1.61–6.97, p = 0.001), CHF (OR = 2.81, 95% CI: 1.39–5.69, p = 0.004) and WBC > 10 × 109/l (OR = 2.96, 95% CI: 1.36–6.42, p = 0.006) were independent risk factors associated with in-hospital mortality. We assigned a score to each variable based on its OR value, with a score of 1 for each indicator, yielding a total score of 5 for the new predictive score for in-hospital mortality in patients with severe AECOPD.
Table III
Logistic regression analysis of in-hospital mortality of patients with severe AECOPD in development cohort
[i] BMI – body mass index, CHF – chronic heart failure, CKD – chronic kidney disease, GER – gastroesophageal reflux, SAHS – sleep apnea hypopnea syndrome, NLR – neutrophil/lymphocyte ratio, PLR – platelet/lymphocyte ratio, CRP – C-reactive protein, NT-proBNP – N-terminal pro-brain natriuretic peptide, PaO2 – arterial partial pressure of oxygen, PaCO2 – arterial partial pressure of carbon dioxide, PaO2/FiO2 – oxygenation index.
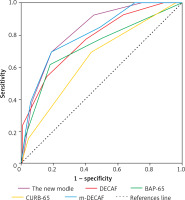
Table IV and Figure 1 show the predictive efficacy of the new score compared to the other predictive scores. In the validation cohort, the area under the ROC curve for the new predictive score was 0.826 (95% CI: 0.724–0.92), which was higher than that for the DECAF score (AUC = 0.783, p = 0.001), BAP-65 score (AUC = 0.730, p = 0.006), CURB-65 score (AUC = 0.652, p = 0.071) and m-DECAF score (AUC = 0.807, p < 0.001).
Table IV
AUROC curve of in-hospital mortality of severe AECOPD patients according to the new score and other scores in validation cohort. AUROC, area under the ROC curve
Discussion
We developed a predictive score to predict in-hospital death in patients with severe AECOPD. The score contains parameters that are simple and easy to obtain and has good predictive power for identification. The new predictive score contains five variables: age > 80 years, confusion, lymphocyte count < 0.8 ×109/l, CHF, WBC > 10 × 109/l.
Internationally, several predictive scores have been developed for mortality during hospitalization in patients with AECOPD, and the scores that have been recognized as good predictors include the DECAF, m-DECAF, BAP-65, and CURB-65. The DECAF score consists of five variables and is commonly used in the general population of patients with AECOPD, while the m-DECAF score replaces one of the variables in the DECAF score with a variable from the DECAF score. A meta-analysis showed that these two scores were superior to other scores in predicting in-hospital mortality in patients with AECOPD [15]. In our study, the new score was a stronger predictor of in-hospital mortality than DECAF and m-DECAF in patients with severe AECOPD. The CURB-65 score contains five variables and is easy to use clinically but is more commonly used in patients with community-acquired pneumonia. The BAP-65 score contains four variables that can be used to predict morbidity and mortality and the need for mechanical ventilation in patients with AECOPD. However, due to the greater complexity of patients with severe AECOPD, the indicators in the above scores are not fully applicable, and some of the indicators were not significantly associated with in-hospital mortality in this study. There are few predictive scores for mortality during hospitalization in patients with severe AECOPD. Therefore, we established a new score to predict the in-hospital mortality of patients with severe AECOPD to help clinical treatment and improve patient prognosis. Meanwhile, we compared the new predictive score with other predictive scores in the validation cohort and found that the new score had better predictive ability. The five variables in the new score are all routine and simple variables obtained during hospitalization and do not require complex calculation formulas, so it is easier to predict patients’ conditions and prognosis, and it is more convenient and quicker to use in the clinic.
Increasing age leads to a physiological decline in lung function, reduced lung remodeling and regenerative capacity, and increased susceptibility to acute and chronic lung disease in the elderly population [16]. Various studies have shown that older age is an important risk factor for poor prognosis in COPD [2, 17]. A multicenter, large-sample retrospective study in China showed that age > 80 years was an independent risk factor for in-hospital death in patients with acute exacerbation of chronic obstructive pulmonary disease [18]. The results of a prospective study showed that old age is a determinant of poor prognosis in patients with AECOPD, with 68–76 years (HR = 6.6; 95% CI: 1.5–28.8, p = 0.013) and ≥ 76 years (HR = 7.2; 95% CI: 1.6–32.6, p = 0.010) being independent predictors of short-term mortality in patients with AECOPD [19].
Our study showed that a significantly higher proportion of severe AECOPD patients who died in hospital had significantly higher rates of impaired consciousness during hospitalization than the surviving group. This is because patients with severe AECOPD often suffer from carbon dioxide retention and reduced partial pressure of oxygen. This may lead to damage to nerve cells in the brain tissue and affect excitability of the cerebral cortex, thus causing altered mental status. Previous studies have found that a low Glasgow Coma Scale (GCS) score is significantly associated with increased mortality in patients admitted to the ICU [20, 21]. Meanwhile, altered consciousness was used as a variable in some of the AECOPD in-hospital mortality prediction scores, such as BAP-65 and CURB-65.
In our previous study, lymphopenia was an independent predictor of in-hospital mortality in patients admitted to the ICU [22]. Since patients with acute exacerbation of chronic obstructive pulmonary disease are often associated with bacterial or viral infections, this affects the balance of the immune system in the lungs, which activates the inflammatory response and leads to infiltration of inflammatory cells in the airways [23, 24], which may lead to a decrease in circulating lymphocytes. Lower lymphocytes mean that patients are less immune and more susceptible to infection, increasing in-hospital mortality. Acanfora found that a low relative lymphocyte count was associated with higher mortality in elderly patients with severe AECOPD [25].
Our study also found that elevated WBC was an independent predictor of in-hospital mortality in patients with severe AECOPD. Elevated WBCs may indicate that AECOPD patients have inflammation and bacterial infections, which can lead to a breakdown of the patient’s immune system, resulting in increased mortality in AECOPD patients. Mia Moberg found that increased leukocytes were a significant predictor of mortality and hospitalization rates in patients with severe AECOPD [26]. In previous studies, leukocytes were significantly higher in AECOPD patients in the death group than in those in the survivor group [27].
About 30% of patients with severe AECOPD in this study population had comorbid chronic heart failure. Chronic obstructive pulmonary disease and chronic heart failure share common risk factors, such as smoking, air pollution, and aging. Chronic heart failure and chronic obstructive pulmonary disease have a mutually reinforcing role in the development of the disease. With the development of chronic obstructive pulmonary disease, the gradual increase in pulmonary vascular resistance leads to increased pulmonary artery pressure and right ventricular dysfunction. In addition, the hypoxia and acidosis caused by chronic obstructive pulmonary disease shorten diastolic and systolic phases, which in turn leads to cardiac dysfunction [28, 29]. It has been found that patients with COPD who have heart failure have an increased mortality rate during hospitalization, and that the all-cause mortality rate of patients with chronic obstructive pulmonary disease who have combined heart failure is 1.6 times higher than that of patients who do not have combined heart failure [30]. One other study found that heart failure was an independent risk factor for long-term mortality in patients with AECOPD [31].
Overall, our study established a simple and practical scoring system for assessing in-hospital mortality in patients with severe AECOPD, which is based on easily accessible history and blood parameters, is low-cost, and has high value for use in clinical care. Our study has some limitations. First, due to its single-center design and small sample size, there may be some degree of selection bias. Second, internal validation at the same center may lead to overfitting, and its applicability to other regions and populations remains to be verified. Thirdly, the prevalence of COVID-19 also affects patient follow-up. Therefore, the score must be validated in a multicenter database with a larger sample size and long-term follow-up in order to evaluate and further improve the score.
In conclusion, we developed a predictive score for predicting mortality during hospitalization in patients with severe AECOPD, which contains five variables: age > 80 years, confusion, lymphocyte count < 0.8 × 109/l, WBC > 10 × 109/l and CHF. The new predictive score can help clinicians assess the prognosis of patients and select appropriate treatment options, which still need to be validated in a larger population in the future.