Introduction
The evaluation of the prognostic criteria in malignant diseases is crucial for establishing an effective induction and consolidation therapy [1]. The responsiveness to chemotherapy and the achievement of complete remission after the first round of treatment are important prognostic factors that may predict the progression of acute myeloid leukemia (AML) [2]. Adverse prognosis may determine poor responsiveness to aggressive therapeutic regimens with no achievement of complete remission and a high risk of relapse, which is the leading causative of mortality among AML patients [3].
Neovascularization is an essential requirement for tumor progression and is initiated by an “angiogenic switch” with consecutive repetitive rounds of destabilization of preexisting vessels, sprouting, maturation, and stabilization of newly formed blood vessels [4]. Oncogenic vascularity promotes malignant cell proliferation, progression, and metastatic spread [5]. However, it may also expose malignant tissue to chemotherapeutic agents [6].
The angiogenic switch is characterized by enhanced localized vascularity as a consequence of an impaired balance of the autocrine and paracrine activities of proangiogenic and antiangiogenic factors [7–9]. The most potent angiogenic promotors of tumors are angiopoietins (Ang), vascular endothelial growth factors (VEGF), and basic fibroblast growth factor (bFGF) [5]. Angiopoietin-1 (Ang-1) is a constitutive inducer of the stability and maturity of blood vessels while angiopoietin-2 (Ang-2) is a context-specific promotor of vascular destabilization and remodeling [10, 11]. Vascular endothelial growth factor-A (VEGF-A) is mainly a hypoxia-inducer of vascular angiogenic factor, while VEGF-C is primarily a lymphangiogenic factor [12]. bFGF promotes angiogenesis by direct enhancement of the proliferation and migration of vascular endothelial cells or by regulation of VEGF by vascular smooth muscle cells [13].
Oncogenic vascularity promotes malignant cell proliferation, progression, and metastatic spread. Considering the essential impacts of the dysregulation of angiogenic factors in tumor progression and metastatic efficacy, they were emphasized in investigating the clinical implications and management of a variety of malignant diseases [14]. The overexpression of angiogenic activators is significantly correlated with the aggressiveness and the poor prognosis of malignant diseases [6].
This study aimed to investigate the modulation of the circulatory levels of Ang-1, Ang-2, bFGF, VEGF-A, and VEGF-C, in patients with AML. The main aim of the study was to evaluate the usefulness of the circulatory level of angiogenic factors as prognostic predictors of patients’ responsiveness to chemotherapy, achievement of remission, and relapsing status.
Material and methods
Patients and sample collection
The study subjects were twenty-four AML patients (n = 24) who had previously been diagnosed with the disease in accordance with the French-American-British (FAB) diagnostic criteria. Based on data that were obtained from their medical profiles, subjects were categorized based on their clinical criteria including relapse status, response to chemotherapy, and achievement of remission. For comparative purposes, an age- and sex-matched control group of fifteen healthy subjects with no hematological diseases was included.
Under aseptic conditions, 5 ml plain blood samples were withdrawn from participants by venipuncture. Blood samples were allowed to clot, after which serum samples were obtained by centrifugation at 4500 rpm for 5 min. Serum samples were stored frozen at –80°C until analyzed. It is worth mentioning the superiority of plasma samples for measurement of angiogenic factors, especially VEGF and Ang-1. These angiogenic factors are stored within platelets and are released upon their in-vivo activation. Therefore, the ex-vivo activation of platelets may falsely elevate the circulatory levels of these factors [15].
Prior to their inclusion, all subjects and/or their guardians were informed about the study, and they approved their participation, after which informed consent was obtained. This study was reviewed and approved by the Institutional Review Board (IRB).
Serum levels of Ang-1, Ang-2, bFGF, VEGF-A and VEGF-C
Enzyme-linked immunosorbent assay (ELISA) was used to determine the serum levels of Ang-1, Ang-2, bFGF, VEGF-A, and VEGF-C. Commercially available kits were purchased (R&D systems, MN, USA) and assays were conducted according to the manufacturer’s instructions. Following plate preparation, 100 µl of standards and samples were transferred into the corresponding wells and incubated at room temperature for 2 h. This was followed by a three-times washing step after which 100 µl of biotinylated antibody was transferred into each well and incubated for 2 h at room temperature. After a second wash, 100 µl of horseradish-peroxidase-streptavidin was added and incubated for 20 min at room temperature. The plate was washed for a third time, after which 100 µl of substrate solution was added and incubated in the dark at room temperature for 20 min. Finally, 50 µl of stop solution was added. The absorbance was measured, using a microplate reader, at a wavelength (λ) of 450 nm.
Statistical analysis
Obtained data were analyzed using version 23 of SPSS Statistics. Results of the descriptive analysis were presented as mean ± standard error mean (SEM). Student’s t-test and analysis of variance (ANOVA) were used for comparative analysis. Correlation analysis was conducted using nonparametric Spearman’s (rho) correlation analysis. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the prognostic value of growth factors against prognostic markers. Statistical significance was defined when the p-value was less than 0.05. Graphs were prepared using GraphPad Prism 6 software.
Results
AML patients had an average age of 38.4 ±22.5 years with a male-to-female ratio of 1.3: 1.0 (n/n is 14: 11). The 15 (n = 15) control subjects had an average age of 38.7±14.4 years and a male-to-female ratio of 1.2: 1.0 (n/n is 8: 7). Nineteen patients (n = 19) were nascent de-novo AML patients while 6 patients (n = 5) were relapsed patients. Thirteen (n = 13) patients were defined to have poor responsiveness to the chemotherapeutic regimen and twelve patients (n = 11) had good responsiveness. Regarding their remission status, fourteen patients (n = 14) failed to achieve remission while eleven patients (n = 10) achieved complete remission.
Comparative analysis of the serum levels of Ang-1, Ang-2, bFGF, VEGF-A, and VEGF-C among AML patients and control subjects are illustrated in Figure 1. Among AML patients, the average serum level of Ang-1 was 59.2 ±12.5 ng/ml which was significantly lower than the corresponding average level among control subjects with 170.8 ±12.7 ng/ml (p < 0.001). The serum level of Ang-2 among AML patients was significantly higher than the corresponding average level among control subjects with average levels of 18.5 ±4.1 ng/ml and 7.5 ±0.8 ng/ml (p < 0.05), respectively. Considering their antagonist relationship, the average ratio of Ang-1 and Ang-2 (sAng-1/sAng-2) among AML patients was 6.8 ±1.8 and was significantly higher than the average ratio of 26.3 ±2.9 among control subjects (p < 0.001).
Figure 1
Comparison of serum levels of angiogenic factors between AML patients (n = 24) and control subjects (n = 15). AML patients had significant dysregulation of Ang-1, Ang-2, Ang-1/Ang-2, and VEGF-A as compared to corresponding levels among control subjects. Results are presented as mean ± SEM ng/ml. *P < 0.05, **p < 0.001
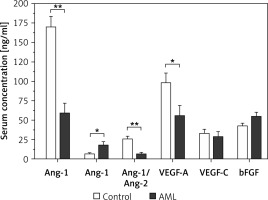
Regarding VEGF-A, AML patients had an average serum level of 56.0 ±13.1 ng/dl, which is significantly lower than the corresponding average serum level among control subjects of 98.6 ±11.9 ng/ml (p < 0.05). There were no significant differences in the serum levels of VEGF-C and bFGF between AML patients and control subjects (p > 0.05). The average serum VEGF-C among AML patients and control subjects were 29.1 ±6.3 ng/ml and 33.7 ±4.6 ng/ml, respectively. The average serum level of bFGF among AML patients was 43.0 ±3.1 ng/ml as compared to 55.1 ±5.3 ng/ml among control subjects.
Based on these findings, a ROC curve analysis was conducted to investigate the diagnostic ability of the sAng-1, sAng-2, and sAng-1/sAng-2 in classifying AML patients. The results revealed that the sAng-1 and Ang-1/Ang-2 ratio have significant predictivities of patients with the disease with an area under the curve (AUC) of 0.900 (95% CI: 0.807 to 0.993) and 0.936 (95% CI: 0.859 to 1.000), respectively (p < 0.001).
A comparative analysis was conducted on the serum levels of s-Ang-1, sAng-2, sAng-1/sAng-2, and VEGF-A among AML patients concerning their clinical status as defined by their relapse status, response to chemotherapy, and achievement of remission. Regarding the relapse status, Figure 2 demonstrates that relapsed AML patients had an average level of sAng-1 of 20.1 ±9.8 ng/ml, which is significantly lower than the corresponding average level of 69.4 ±14.9 ng/ml (p < 0.05) among non-relapsed-patients. No significant difference in the serum levels of Ang-2 was observed: relapsed patients had an average level of 11.7 ±2.4 ng/ml as compared to an average of 20.3 ±5.0 ng/ml among non-relapsed patients. Regarding Ang-1/Ang-2, relapsed AML had a significantly lower average ratio of 1.7 ±0.9 as compared to an average ratio of 8.1 ±2.2 (p < 0.05) among non-relapsed patients. The differences in the serum levels of VEGF-A, VEGF-C, and bFGF are statistically insignificant.
Figure 2
Comparison of serum levels of angiogenic factors among relapsed AML patients (n = 5) and non-relapsed patients (n = 19). Relapsed patients had significant downregulation of serum Ang-1 and Ang-1/Ang-2 levels. Results are presented as mean ± SEM ng/ml. *P < 0.05
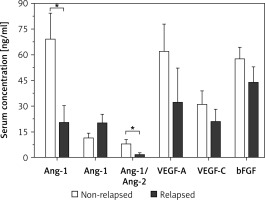
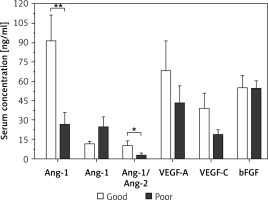
Figure 3 presents the results of the comparative analysis of the serum levels of angiogenic factors with patients’ responsiveness to chemotherapy. Patients with poor responsiveness had a significantly lower average level of sAng-1 of 26.8 ±9.2 ng/ml as compared to an average level of 91.7 ±19.5 ng/ml among patients with good responsiveness (p < 0.01). Regarding Ang-2, the levels among patients with poor response with an average of 25.0 ±7.5 ng/ml are not significantly different from the corresponding levels among poorly responsive patients with an average of 11.9 ±1.7 ng/ml. The ratio of sAng-1/sAng-2 was significantly lower among patients with poor responsiveness compared to those with good responsiveness with an average ratio of 2.9 ±1.6 and 10.6 ±2.9, respectively (p < 0.05). There were no significant differences in the average serum levels of VEGF-A, VEGF-C, and bFGF between the two groups of patients.
Figure 3
Comparison of serum levels of angiogenic factors between AML patients with good (n = 11) and poor (n = 13) responsiveness to chemotherapy. Patients with poor response had significant downregulation of serum Ang-1 and Ang-1/Ang-2 as compared to patients with good responsiveness. Results are presented as mean ± SEM ng/ml. *P < 0.05, **p < 0.001
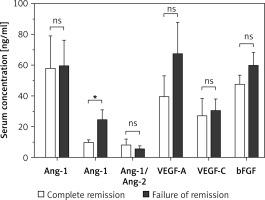
Results of comparative analysis between patients who achieved complete remission and patients with failed achievement of remission are illustrated in Figure 4. Patients with failed remission had an average serum Ang-2 level of 24.6 ±6.4 ng/ml, which is significantly higher than the corresponding average among patients with complete remission of 9.9 ±1.5 ng/ml (p < 0.05). Between the two groups, there were no significant differences in the average serum levels of Ang-1, Ang-1/Ang-2, VEGF-A, VEGF-C, and bFGF.
Figure 4
Comparison of serum levels of angiogenic factors among AML patients who achieved complete remission (n = 10) and patients with failed achievement of remission (n = 14). Patients with failed remission had significantly higher average serum levels of Ang-2 as compared to the corresponding average level among patients with complete remission. Results are presented as mean ± SEM ng/ml. *P < 0.05
To investigate the association between the assigned growth factors and the clinical status of AML patients, Spearman’s correlation analysis was conducted, and the results are illustrated in Table I. No significant association between the serum levels of investigated angiogenic factors and the relapsing status of patients was found (p > 0.05). However, the status of poor responsiveness to chemotherapy showed a significant association with the decrease in the serum levels of Ang-1 (p < 0.05). Regarding the status of achieving remission, the extent of upregulation in the serum levels of Ang-2 had a significant association with the unfavorable failed remission status (p < 0.05).
Table I
Spearman’s (rho) correlation analysis of serum levels of investigated angiogenic factors and the clinical status of patients in terms of relapse status, responsiveness to chemotherapy, and status of remission achievement
| Angiogenic factor | Spearman’s corr. | Relapse status | Response to therapy | Remission status |
|---|---|---|---|---|
| Ang-1 | rho (ρ) | 0.274 | 0.488* | 0.055 |
| P-value | 0.77 | 0.016 | 0.799 | |
| Ang-2 | rho (ρ) | 0.126 | 0.229 | 0.476* |
| P-value | 0.56 | 0.28 | 0.019 | |
| Ang-1/Ang-2 | rho (ρ) | 0.230 | 0.445* | 0.049 |
| P-value | 0.28 | 0.029 | 0.821 | |
| bFGF | rho (ρ) | 0.289 | 0.108 | 0.196 |
| P-value | 0.17 | 0.614 | 0.360 | |
| VEGF-A | rho (ρ) | 0.200 | 0.217 | 0.195 |
| P-value | 0.35 | 0.309 | 0.360 | |
| VEGF-C | rho (ρ) | 0.067 | 0.205 | 0.214 |
| P-value | 0.76 | 0.337 | 0.316 |
Considering the findings of comparative and correlation analysis, the predictive value of serum angiopoietins of the clinical status of AML patients was further evaluated by ROC curve analysis. Regarding the predictivity of poor patients’ responsiveness to the chemotherapeutic regimen, the AUC values of sAng-1, sAng-2, and sAng-1/Ang-2 were 0.781 (95% CI: 0.581 to 0.982, p = 0.019), 0.632 (95% CI: 0.403 to 0.861, p = 0.273), and 0.757 (95% CI: 0.547 to 0.967, p = 0.033), respectively.
The predictivity of sAng-1, sAng-2, and sAng-1/sAng2 of the incomplete achievement of remission was demonstrated by AUC of 0.468 (95% CI: 0.226 to 0.709, p = 0.792), 0.779 (95% CI: 0.592 to 0.965, p = 0.022) and 0.529 (95% CI: 0.286 to 0.771, p = 0.815). No discriminative ability of serum levels of angiopoietin for patients’ relapse status was found. The AUC for sAng-1 was 0.695 (95% CI: 0.481 to 0.909, p = 0.109), for sAng-2 was 0.411 (95% CI: 0.174 to 0.648, p = 0.546), and for sAng-1/sAng-2 was 0.663 (95% CI: 0.447 to 0.879, p = 0.110).
Discussion
Tumor neovascularization depends on the dysregulation of the balanced autocrine and paracrine activities of pro-angiogenic and anti-angiogenic factors [9]. Herein, the significant dysregulation of the serum levels of Ang-1, Ang-2, and VEGF-A is definitive of their contribution to the pathogenesis of the disease. This contribution is supported by the significant diagnostic predictivity of sAng-1 downregulation and sAng-2 upregulation.
Whilst Ang-1, Ang-2, and VEGF-A can act in a paracrine manner on endothelial cells, bFGF and VEGF-C act in an autocrine manner [16, 17]. It has been demonstrated that the autocrine activities of bFGF and VEGF-C in the bone marrow of AML patients promote tumor progression and predict adverse clinical outcomes of the disease [18–20]. Therefore, the trivial dysregulations in the serum levels of bFGF and VEGF-C in patients may not indicate the lack of their contribution to the pathogenicity of the disease and can be explained by their autocrine activities that limit their release in the circulation.
The antagonist activities of Ang-1 and Ang-2 explain their inverse dysregulation in opposite directions. In the context of chronic conditions such as coronary artery disease and diabetes mellitus, the antagonist dysregulation of Ang-1 and Ang-2 destabilizes vascular endothelium and subsequently promotes vascular atherosclerosis [21]. The downregulation of Ang-1 and upregulation of Ang-2 are suggestive of antiangiogenic properties that render downstream signaling to enhance vascular destabilization to promote either sprouting or regression [22, 23]. Following the angiogenic switch in tumors, this angiopoietin dysregulation induces unidirectional sprouting in the presence of VEGF [24, 25]. However, if VEGF is downregulated, vascular regression is evident [25, 26]. It has been shown that enhanced systemic expression of Ang-2 causes vascular regression regardless of VEGF levels [27]. Accordingly, our reported serum levels of angiopoietins and VEGF among AML patients are consistent with vascular destabilization and remodeling that precedes a status of vascular regression. Vascular regression has been proposed to be an integral phase of tumorigenesis where cancer encroaches the preexisting vascularity that is followed by destabilization and regression in the center of the tumor mass and then angiogenesis at the tumor periphery is initiated [28]. Furthermore, in response to chemotherapeutic intervention, vascular regression exacerbates hypoxia while improving perfusion of surviving malignant and enables them to avoid apoptotic death by inducing survival signaling pathways [25, 29].
Angiopoietins serve rate-limiting functions in tumor vascularization where their antagonist properties are crucial to maintaining vascular plasticity [27, 30, 31]. The upregulation of sAng-2 level has been linked to promoted tumor size and increased metastatic efficacy [32, 33]. In AML, it has been demonstrated that the overexpression of tissue Ang-2 is associated with an extended overall survival of malignant blasts as compared to blasts with low levels of expression [34]. In patients with multiple myeloma, marked upregulation of the serum level of Ang-2 is a hallmark among patients with advanced stages of the disease and is directly correlated with prognostic factors of the disease including lytic bone lesions and serum β2-microglobulin level [35].
Therefore, it is reasonable to propose the connection between serum angiopoietin levels and the clinical status of patients concerning their responsiveness to chemotherapy and achievement of remission. Principally, this study aimed to evaluate the utilization of the serum levels of concerned angiogenic factors to predict AML patients’ responsiveness to chemotherapy, achievement of remission, and the risk of relapse. We detected significant associations between the dysregulation of serum levels of Ang-1 and Ang-2 with patients’ responsiveness to chemotherapy and achievement of remission, respectively. These associations were endorsed by good predictivity of sAng-1 downregulation of poor responsiveness to chemotherapy and by good predictivity of sAng-2 upregulation of the status of remission failure.
Considering their interrelatedness, the relative ratio of sAng-1 to Ang-2 has been defined as a more reliable diagnostic and prognostic marker for several adverse clinical outcomes of malignant diseases including cervical cancer, ovarian cancer, colorectal cancer, melanoma, and hepatocellular carcinoma [10]. The downregulation of sAng-1/sAng-2 is significantly associated with good predictivity of patients’ poor responsiveness to chemotherapy but not with relapse status or achievement of remission status.
In conclusion, downregulation of sAng-1 and upregulation of sAng-2 may serve as useful prognostic tools to predict patients’ responsiveness to chemotherapy and the status of achieving remission, respectively. This study has potential limitations due to which our findings should be considered preliminary, requiring further investigation to enhance their validity and clinical reliability. The first limitation concerns the small sample size and the single time-point measurement throughout the therapeutic intervention. A larger sample size with multiple time-point measurements should make it possible to conduct multivariate analysis and survival analysis to validate the reliability and predictivity of clinical outcomes. Secondly, there is a lack of molecular and cytogenetic features that are established as important diagnostic and prognostic hallmarks to predict the clinical outcomes of both de novo and relapsed AML [3, 36]. Thirdly, there is a lack of data to define the intensiveness (dosage, frequency, and duration) of the stratified chemotherapeutic regimen, which varies among patients in accordance with their disease progression [37]. The final limitation concerns the unavailability of demographic features, which vary among patients and affect their clinical status at the time of diagnosis, including the percentage of bone marrow blasts, peripheral blood leukocytosis, FAB classification, and the duration of disease-free survival of relapsed patients.