Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ORTHOPEDICS AND TRAUMATOLOGY / CLINICAL RESEARCH
Research based on nucleotide polymorphism reveals the role of inflammatory cytokines in regulating the influence of blood metabolites on drug-related osteonecrosis
1
Clinical Medical College, Hebei University of Engineering, Handan, China
2
Institute of Orthopedics, The Fourth Medical Center of Chinese PLA General Hospital, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Beijing, China
Submission date: 2024-11-22
Final revision date: 2025-01-27
Acceptance date: 2025-01-31
Online publication date: 2025-04-01
Corresponding author
Jiang Peng
Institute of Orthopedics, The Fourth Medical Center of Chinese PLA General Hospital No. 51 Fucheng Road Beijing, China
Institute of Orthopedics, The Fourth Medical Center of Chinese PLA General Hospital No. 51 Fucheng Road Beijing, China
KEYWORDS
nucleotide polymorphismpro-inflammatory mediatorsmetabolitesdrug-related osteonecrosisMendelian randomization
TOPICS
ABSTRACT
Introduction:
Osteonecrosis is a debilitating disease caused by impaired blood supply leading to bone tissue death, and drug-related osteonecrosis is a significant clinical issue. The role of inflammation and metabolic disorders in the pathogenesis of osteonecrosis has garnered widespread attention, but the exact causal relationships remain unclear. This study aims to explore the causal link between inflammatory cytokines and drug-related osteonecrosis, while also investigating how metabolites might mediate this relationship.
Material and methods:
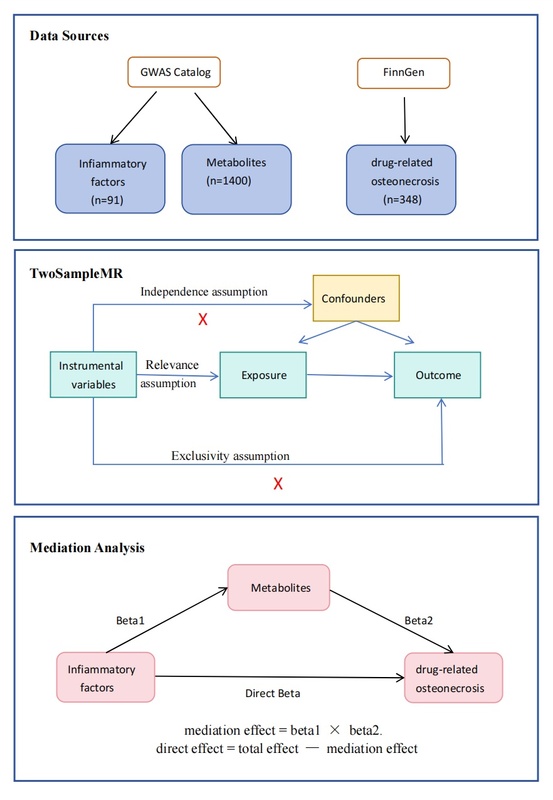
We employed two-sample Mendelian randomization (MR) analysis to examine the causal links between 91 inflammatory cytokines, 1,400 blood metabolites, and drug-related osteonecrosis. Single nucleotide polymorphisms (SNPs) associated with inflammatory cytokines and metabolites were used as instrumental variables (IVs) to assess their potential relationship with drug-related osteonecrosis risk. We further conducted mediation MR analysis to explore the role of metabolites in mediating the impact of inflammatory cytokines on drug-related osteonecrosis.
Results:
MR analysis demonstrated notable causal relationships between four inflammatory cytokines and drug-related osteonecrosis. Specifically, interleukin-4 (IL-4) and C-X-C motif chemokine 6 (CXCL6) showed a negative correlation with the risk of drug-related osteonecrosis, while interleukin-6 (IL-6) and glial cell line-derived neurotrophic factor (GDNF) exhibited a positive correlation with the risk. Furthermore, mediation analysis revealed that IL-4 affects the development of drug-related osteonecrosis via blood metabolites. Key metabolites identified as significant mediators included mannitol/sorbitol levels, the mannose-to-mannitol-to-sorbitol ratio, and the glucose-to-mannitol-to-sorbitol ratio.
Conclusions:
This study presents new evidence connecting inflammatory cytokines to drug-related osteonecrosis and highlights the mediating role of metabolites. These results help us understand the pathogenesis of the disease and provide new insights for its prevention and treatment.
Osteonecrosis is a debilitating disease caused by impaired blood supply leading to bone tissue death, and drug-related osteonecrosis is a significant clinical issue. The role of inflammation and metabolic disorders in the pathogenesis of osteonecrosis has garnered widespread attention, but the exact causal relationships remain unclear. This study aims to explore the causal link between inflammatory cytokines and drug-related osteonecrosis, while also investigating how metabolites might mediate this relationship.
Material and methods:
We employed two-sample Mendelian randomization (MR) analysis to examine the causal links between 91 inflammatory cytokines, 1,400 blood metabolites, and drug-related osteonecrosis. Single nucleotide polymorphisms (SNPs) associated with inflammatory cytokines and metabolites were used as instrumental variables (IVs) to assess their potential relationship with drug-related osteonecrosis risk. We further conducted mediation MR analysis to explore the role of metabolites in mediating the impact of inflammatory cytokines on drug-related osteonecrosis.
Results:
MR analysis demonstrated notable causal relationships between four inflammatory cytokines and drug-related osteonecrosis. Specifically, interleukin-4 (IL-4) and C-X-C motif chemokine 6 (CXCL6) showed a negative correlation with the risk of drug-related osteonecrosis, while interleukin-6 (IL-6) and glial cell line-derived neurotrophic factor (GDNF) exhibited a positive correlation with the risk. Furthermore, mediation analysis revealed that IL-4 affects the development of drug-related osteonecrosis via blood metabolites. Key metabolites identified as significant mediators included mannitol/sorbitol levels, the mannose-to-mannitol-to-sorbitol ratio, and the glucose-to-mannitol-to-sorbitol ratio.
Conclusions:
This study presents new evidence connecting inflammatory cytokines to drug-related osteonecrosis and highlights the mediating role of metabolites. These results help us understand the pathogenesis of the disease and provide new insights for its prevention and treatment.
REFERENCES (36)
1.
Petek D, Hannouche D, Suva D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev 2019; 4: 85-97.
2.
Zhu T, Cui Y, Zhang M, Zhao D, Liu G, Ding J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact Mater 2020; 5: 584-601.
3.
Li Z, Yang B, Weng X, Tse G, Chan MTV, Wu WKK. Emerging roles of MicroRNAs in osteonecrosis of the femoral head. Cell Prolif 2018; 51: e12405.
4.
Gupta A, Tripathi L, Pandey S, Dwivedi D. Biology of bone morphogenetic proteins in skeleton disease: osteonecrosis in sickle cell disease patients. Curr Protein Pept Sci 2022; 23: 264-70.
5.
Li L, Zhao S, Leng Z, et al. Pathological mechanisms and related markers of steroid-induced osteonecrosis of the femoral head. Ann Med 2024; 56: 2416070.
6.
Murphey MD, Foreman KL, Klassen-Fischer MK, Fox MG, Chung EM, Kransdorf MJ. From the radiologic pathology archives imaging of osteonecrosis: radiologic-pathologic correlation. Radiographics 2014; 34: 1003-28.
7.
Chen H, Xu J, Lv Y, et al. Proanthocyanidins exert a neuroprotective effect via ROS/JNK signaling in MPTP induced Parkinson’s disease models in vitro and in vivo. Mol Med Rep 2018; 18: 4913-21.
9.
Wu X, Xu W, Feng X, et al. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int J Immunopathol Pharmacol 2015; 28: 351-61.
10.
Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone 2016; 6: 119-30.
11.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13: 159-75.
12.
Zhu D, Yu H, Liu P, et al. Calycosin modulates inflammation via suppressing TLR4/NF-κB pathway and promotes bone formation to ameliorate glucocorticoid-induced osteonecrosis of the femoral head in rat. Hytother Res 2021; 35: 2824-2835.
13.
Yu X, Zhang S, Zhang B, Dai M. Relationship of idiopathic femoral head necrosis with blood lipid metabolism and coagulation function: a propensity score-based analysis. Front Surg 2023; 9: 938565.
14.
Zhu W, Wang R, Yang Z, et al. GC-MS based comparative metabolomic analysis of human cancellous bone reveals the critical role of linoleic acid metabolism in femur head necrosis. Metabolomics 2023; 19: 86.
15.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA 2017; 318: 1925-6.
16.
Zhao JH, Stacey D, Eriksson N, et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol 2023; 24: 1540-51.
17.
Chen Y, Lu T, Pettersson-Kymmer U, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet 2023; 55: 44-53.
18.
Orrù V, Steri M, Sidore C, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet 2020; 52: 1036-45.
19.
Chen J, Yu X, Wu X, Chai K, Wang S. Causal relationships between gut microbiota, immune cell, and non-small cell lung cancer: a two-step, two-sample Mendelian randomization study. J Cancer 2024; 15: 1890-7.
20.
Jiazhen H, Bozhen F. CSF1 mediating 3-hydroxyhexanoate in endometrial cancer: a Mendelian randomization study. Arch Med Sci 2024; 20: 1731-5.
21.
Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 2011; 40: 755-64.
22.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013; 37: 658-65.
23.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 2017; 36: 1783-802.
24.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018; 50: 693-8.
25.
Sekula P, del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 2016; 27: 3253-65.
26.
Carter AR, Sanderson E, Hammerton G, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 2021; 36: 465-78.
27.
Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 2008; 9: 310-8.
28.
Wang SZ, Zhang YL, Shi HB. Potential repressive impact of microRNA-20a on renal tubular damage in diabetic kidney disease by targeting C-X-C motif chemokine ligand 6. Arch Med Res 2021; 52: 58-68.
29.
Xu L, Yu Y, Sang R, Li J, Ge B, Zhang X. Protective effects of taraxasterol against ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF-B signaling pathways in mice. Oxid Med Cell Longev 2018; 2018: 8284107.
31.
Sims NA. Cell-specific paracrine actions of IL-6 family cytokines from bone, marrow and muscle that control bone formation and resorption. Int J Biochem Cell Biol 2016; 79: 14-23.
32.
Yamada J, Akeda K, Sano T, Iwasaki T, Takegami N, Sudo A. Expression of glial cell line-derived neurotrophic factor in the human intervertebral disc. Spine 2020; 45: E768-75.
33.
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813-20.
34.
Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc 2019; 8: e012673.
35.
Kizer JR, Benkeser D, Arnold AM, et al. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis 2014; 235: 116-21.
36.
Lamprea-Montealegre JA, Arnold AM, McCLelland R, et al. Plasma levels of advanced glycation endproducts and risk of cardiovascular events: findings from 2 prospective cohorts. J Am Heart Assoc 2022; 11: e024012.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.