Introduction
Cardiovascular diseases (CVD) still account for 45% of all deaths worldwide [1, 2]. The underlying cause of many CVD is coronary atherosclerosis, a highly complex entity characterized by chronic, low-grade inflammation and occlusive plaque formation that leads to inadequate oxygen supply of the myocardium [3]. A common coronary artery disease (CAD) manifestation is acute coronary syndrome (ACS), which comprises unstable angina pectoris (AP), acute myocardial infarction (AMI) without ST-segment elevation (NSTEMI) and ST-segment elevation myocardial infarction (STEMI) [4]. According to the Vidal-Perez research group, between 5% and 25% of all myocardial infarctions are myocardial infarction with non-obstructive coronary arteries (MINOCA) [5].
Telomere deoxyribonucleic acid (DNA) is composed of repeating, non-coding, hexameric sequences d(TTAGGG) that protect chromosome end-points from end-to-end fusion and degradation, prevent loss of genetic information and provide genome stability [6]. Telomere length and integrity are regulated through the activity of telomerase enzyme [6], a ribonucleic complex with enzymatic activity that adds short, repeating sequences d(TTAGGG) on the 3′-end of telomere DNA, thus prolonging telomeres and the lifespan of cells [7].
A close connection between oxidative stress and CVD has been established [8, 9]. Oxidative stress might cause oxidative damage of DNA, including telomeres, leading to their shortening, although the precise mechanism is not well understood [10, 11]. A number of sources of evidence suggest that telomere dysfunction might be a key molecular event in the pathogenesis of CVD, although most studies were performed on in vitro models, and there is still a lack results confirming this hypothesis on human samples [12]. Likewise, oxidative stress is thought to impact telomerase activity causing its deactivation, but the direct link with telomerase structure damage remains unclear. Still, there are conflicting conclusions on this topic, and there are limited results for a complex human medium like peripheral blood [13–15].
The aim of this study was to evaluate telomere length and telomerase activity in human samples, i.e. circulating leukocytes and thromboaspirates of patients with AMI. Comparing the elements of the telomere-telomerase system in separate cardiovascular entities was performed in order to better understand the stages of CVD progression. The elements of the telomere-telomerase system were also analysed to associate them with oxidative stress markers and evaluate possible use of mentioned circulating parameters as diagnostic or prognostic markers in AMI patients.
Material and methods
Experimental design
The study was conducted from 2015 to 2016, and patients were followed for the next 4 years for the outcome (until December 2019). Patients with acute myocardial infarction were selected from the patients admitted to the intensive care units of Clinical Hospital Center Zemun and Clinical Hospital Center “Bezanijska kosa”. Inclusion criteria were: patients with acute myocardial infarction with STEMI from 18 to 80 years old of both genders, with infarction pain present for a maximum of 12 h and candidates for primary percutaneous coronary intervention. Patients were excluded from the study if they had STEMI caused by thrombosis of the previous stent, received thrombolytic therapy, had STEMI after previous coronary artery bypass graft surgery, cardiogenic shock, pregnant women and those who did not give their consent. Patients were enrolled in the study after they had received appropriate treatment. Since it was not possible to separate MINOCA patients from non-MINOCA patients during selection, we further divided them in three entities: STEMI, MINOCA or patients with AMI but without an implanted stent because of blood vessel rupture (hereinafter null-stent patients (NSP)). In order to better understand the relation of the telomere-telomerase system, oxidative stress and coronary artery disease, we selected AP patients as a group that presents all the cardiovascular risk factors but did not develop AMI. AP patients were recruited among the patients from the cardiology department of Clinical Hospital Center Zemun and Clinical Hospital Center “Bezanijska kosa” with programmed coronary angiography examination.
Parallel with patients, the control group of 84 healthy persons, matched patients according to gender and age, was evaluated. The control group was selected for the study based on the following exclusion criteria: high blood pressure (systolic above 140 mm Hg; diastolic above 90 mm Hg), presence of cardiovascular disease, presence of renal, hepatic or malignant disease or previous infections, trauma and surgery interventions. Additionally, the absence of cardiovascular diseases in the control group was confirmed by electrocardiogram, echocardiogram and cardiac stress test performed by a cardiologist in Clinical Hospital Center Zemun and Clinical Hospital “Bezanijska kosa”.
All the participants were appropriately informed about the purpose and the aim of the study, and signed an informed consent form before they were included in the research. The Ethical Committees of Clinical Hospital Center Zemun (n. 325/1, from September 24th 2015), Clinical Hospital Center “Bezanijska kosa” (n. 4705/4, from May 31st 2016 and General hospital “Medigroup” (n. 1080/15, from June 18th 2016) approved this study protocol.
Sample collection
Blood samples from the patients were obtained: i) upon admission to the Emergency centre, before the primary percutaneous coronary intervention (pPCI) procedure, for the first time point, ii) after the pPCI procedure was finished, for the second time point, and iii) 6 months after the acute event and after overnight fasting, for the third time point.
We collected peripheral venous blood (i.e. serum, plasma and whole blood samples (i.e. for telomerase enzyme and DNA isolation)) at indicated time points. In addition, aspirated occlusive lesions (thromboaspirates) were collected when indicated, during the pPCI procedure. The peripheral blood samples were obtained in the control group after overnight fasting.
Assays
Telomerase enzyme activity was measured using modified Real-Time Telomeric Repeat Amplification Protocol (RTq-TRAP) as described in our previous work [16]. Briefly, total leukocytes were collected from the whole blood, resuspended in ice cold CHAPS buffer, incubated for 30 min on ice and afterwards centrifuged for 20 min at 16,000 g. The supernatant was collected for further analysis. The telomerase activity was measured using primers TS: 5′-AATCCGTCGAGCAGAGTT-3′ and ACX: 5′-GCGCGGCTTACCCTTACCCTTACCCTAACC-3′ in 10 µL of the final mix containing qPCR Master Mix (5x HOT FIREPol EvaGreen qPCRSuperMix, Solis Biodyne, Tartu, Estonia), TS primer, ACX primer and protein extract. PCR amplification followed the protocol: i) telomerase extension reaction for 30 min at 25ºC, ii) 95°C for 12 min, iii) 50 cycles of 95°C for 15 s, 60°C for 15 s, 72°C for 35 s, and iv) dissociation curve with settings 95°C for 1 s, 50°C for 1 s, 95°C continuously. The telomerase activity was calculated according to the formula by Elmore et al. [17].
Leukocyte telomere length (LTL) was determined with modified qPCR and calculated as T/S ratio [18]. LTL was determined with modified qPCR [19, 20]. In short, genomic DNA was extracted using a commercial DNA kit (Flexi GENE DNA kit, Qiagen). We opted for albumin as a single copy reference gene. The primers used in PCR reaction were: Tel-forward (5′-ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT-3′), Tel reverse (5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-3′), Alb forward (5′-CGGCGGCGGGCGGCGCGGGCTGGGCGGAAATGCTGCACAGAATCCT TG-3′) and Alb reverse (5′-GCCCGGCCCGCCGCGCCCGTCCCGCCGGAAAAGCATGGTCGCCTGTT-3′). The final reaction mixtures for the telomere length and the single copy reference gene (albumin) contained: qPCR Master Mix (5x HOT FIREPol EvaGreen qPCR SuperMix, Solis Biodyne, Tartu, Estonia), forward primer, reverse primer and DNA template. The thermal cycling profile for the telomere length included the following steps: i) 95 ºC for 12 min, ii) 4 cycles of 95°C for 15 s, 49°C for 20 s, iii) 40 cycles of 95°C for 15 s, 62°C for 10 s, 72°C for 15 s, and iv) dissociation curve with settings 95°C for 1 s, 50°C for 1 s, 95°C continuously. The PCR profile for albumin amplification contained the following steps: i) 95°C for 12 min, ii) 40 cycles of 95°C for 15 s, 62°C for 10 s, 87°C for 15 s, followed by iv) dissociation curve with settings 95°C for 1 s, 50°C for 1 s, 95°C continuously. PCR measurements for both the telomerase activity and telomere length were carried out on the LighCycler 480 II System (F. Hoffmann-La Roche Ltd, Basel, Switzerland). Until now, there is no evidence for clinical implementation of the mentioned biomarker, although they were strongly suggested [21–23].
The oxidative stress was evaluated through comprehensive scores: Protective, Damage and OXY score suggested by Veglia et al. [24]. In short, the Protective score was calculated as the average z-score of beneficial antioxidant parameters (total antioxidative status, content of sulfhydryl groups, paraoxonase activity, superoxide-dismutase activity), while the Damage score was the average z-score of pro-oxidant markers (pro-oxidative-antioxidative balance, total oxidative status, advanced oxidation protein products). The OXY score was calculated as the difference between average z-scores of Protective and Damage scores calculated as previously explained. The expected value of the OXY score is near zero if levels of pro-oxidant markers are completely compensated by antioxidant defences [19]. All three scores are presented in arbitrary units.
Biochemical parameters (total cholesterol, triglycerides and creatine-kinase activity (CK)) were measured using routine enzymatic methods, while high-density lipoprotein cholesterol (HDL-C) was evaluated by the direct enzymatic method on the ILAB 600 analyser (Instrumentation Laboratory, Milan, Italy). To calculate the concentration of low-density lipoprotein cholesterol (LDL-C) the Friedewald formula was used. Levels of troponin I were determined using the commercial Access Immunoassay system (UniCelDxI 600 Access Immunoassay System, Beckman Coulter Inc, USA). All haematology parameters were measured using the ADVIA haematology system (Siemens Healthcare GmbH, Erlangen, Germany).
Statistical analysis
Normality distribution for all variables was checked by the Kolmogorov-Smirnov test or the Shapiro-Wilk test for groups with less than 50 patients. Data are presented as mean ± standard deviation for normally distributed variables, absolute frequencies for categorical variables or medians with 25th and 75th percentile value for variables with non-normal distribution. Parameters with normal distribution were analysed with ANOVA followed by Tukey’s post-hoc test for the differences in subgroups, while asymmetrically distributed variables were analysed by the Mann-Whitney and Kruskal-Wallis test and frequencies with the chi-square test. Data from different time points were evaluated using Friedman’s test for repeated measures. Parameters with a value of 0 were not included in the comparison. To evaluate the potential impact of certain parameters on each other, multiple regression analysis (forward selection) was used. Potential ability of parameters to distinguish patients from healthy persons was checked by receiver operating characteristic curve (ROC) analysis. All statistical analyses were done using PASW Statistic v.18 (Chicago, Illinois, USA) software. The p-value < 0.05 was set as statistically significant, while the groups with non-parametric distribution were corrected using Bonferroni adjustment and the significance level was p-value < 0.005. All the parameters in Figure 1 and Table I were taken into consideration before Bonferroni corrections. After Bonferroni corrections, parameters with a p-value < 0.005 were not statistically significant.
Table I
Telomere length, telomerase activity and oxidative-stress scores in STEMI patients through the different time points after the acute coronary event in blood and in thromboaspirate
| Parameter | Control subjects (n = 84) | STEMI patients | P-value | |||
|---|---|---|---|---|---|---|
| Acute state (n = 25) | After pPCI intervention (n = 25) | 6-month follow-up (n = 25) | Thromboaspirate (n = 17) | |||
| Telomere length (T/S ratio) | 1.39 (1.110–1.628) | 1.18 (0.909–1.516)a | 1.62 (1.18–2.05)aa,bb | 1.15 (0.93–1.33)a | 1.25 (1.01–1.84)c | 0.036 |
| Telomerase activity (log activity) | 0.069 (0.061–0.081) | 0.359 (0.345–0.394)aa | 0.363 (0.354–0.395)aa | 0.395 (0.367–0.421)aa, b | 0.366 (0.367–0.379)aa | < 0.001 |
| Damage score | 4.62 (3.62–5.49) | 5.71 (4.09–7.87)a | 4.47 (3.70–6.15)b | 4.14 (3.08–5.38)b | 4.81 (2.33–19.93)b | 0.001 |
| Protective score | 0.357 (–0.205–0.885) | 0.363 (0.071–1.189) | –0.313 (–0.67–0.64)a,b | –1.94 (–0.59––3.61)a,b | 12.23 (0.376–28.33)aa, bb | < 0.001 |
| OXY score | 4.79 (3.21–5.84) | 4.99 (4.05–6.73) | 4.69 (3.51–6.13)b | 5.86 (4.71–7.52)a,b | –3.04 (–22.81–46.47)aa,bb | < 0.001 |
aa,bb,cc,dd p < 0.001 vs. control group, acute state, after pPCI intervention respectively. All results are variables with non-normal distribution and are presented as medians with 25th and 75th percentile value. Variables are analysed using Friedman’s test for repeated measures and the Wilcoxon test for paired values. STEMI – ST-segment elevation myocardial infarction, pPCI – primary percutaneous coronary intervention.
Data processing
Telomere length and telomerase activity might be influenced by various factors, such as gender, smoking habit, body mass index (BMI), comorbidity with diabetes mellitus type 2, use of hypolipidemic drugs (e.g., statins) [24–29].
The data with non-normal distribution were first logarithmically transformed (data not shown) and included in the multiple linear regression (forward selection). We started from all the parameters that represent the risk factors for CVD, and the final best model consisted of telomerase activity, total cholesterol, HDL-C, and LDL-C.
A receiver operating characteristic (ROC) analysis of the selected parameters was conducted to test their discriminatory ability regarding healthy population and STEMI patients or STEMI patients and AP patients. After initial ROC analysis we constructed a model of parameters using logistic regression analysis generated predictive probabilities. A cut-off value was calculated using the Youden index (sensitivity + specificity – 1), where the highest calculated Youden index values indicated the corresponding cut-off.
Results
This study included 128 cardiovascular patients. The patient groups included 106 patients admitted with AMI, candidates for the primary percutaneous coronary intervention (pPCI) procedure (among them 93 patients with STEMI, 7 MINOCA patients and 6 patients with AMI without an implanted stent because of blood vessel rupture (further NSP)), and 22 patients with AP selected for angiography analysis. The control group of 84 healthy persons, matched to patients according to gender and age, was evaluated.
The overview of all the participants included in the study and basic patients’ clinical data are summarised in Table II. All groups were similar in age, BMI and diastolic blood pressure. Significantly higher values of systolic blood pressure were noted in the groups of STEMI patients and patients without an implanted stent compared to the control group. The basic biochemical and haematological parameters of the participants included in the study are summarised in Table III.
Table II
Demographic and clinical data of study participants
| Parameter | Control subjects (n = 84) | AP patients (n = 22) | STEMI patients (n = 93) | MINOCA patients (n = 7) | NSP (n = 6) | P-value |
|---|---|---|---|---|---|---|
| Age# [years] | 56 ±5 | 60 ±10 | 61 ±11 | 59 ±13 | 60 ±10 | 0.923 |
| BMI# [kg/m2] | 26.2 ±3.9 | 28.4 ±4.2 | 25.8 ±4.5 | 25.9 ±2.9 | 26.6 ±7.7 | 0.195 |
| Cigarette smoking, yes/no | 16/68 | 6/16 | 67/93aa,bb | 3/4 | 2/4 | < 0.001 |
| Systolic BP# [mm Hg] | 127 ±18 | 131 ±11 | 136 ±24a | 161 ±30 | 127 ±17a | 0.004 |
| Diastolic BP# [mm Hg] | 82 ±10 | 79 ±7 | 85 ±14 | 96 ±26 | 82 ±11 | 0.093 |
| Number of blood vessels with clinical significant stenosis | – | 1–3 | 1–5 | 0 | 1–3 | – |
| Number of implanted stents | – | 0 | 1–3 | 0 | 0 | – |
| Dyslipidaemia, % | – | 40.9a | 45.2a | 42.9a | 33.3a | < 0.001 |
| Statins, % | – | 40.9a | 17.2a | 0 | 0 | < 0.001 |
| Diabetes mellitus, % | – | 0 | 9.7a | 28.6a | 0 | < 0.001 |
| Glucose intolerance, % | – | 36.4a | 8.6a | 57.1a | 0 | < 0.001 |
aa,bb p < 0.001 vs. control, AP, STEMI, MINOCA group. Results are presented as frequencies or mean ± standard deviation for normally distributed variables (#). ANOVA followed by Tukey’s post-hoc test for differences in subgroups was used for normally distributed parameters. Frequencies were analysed with the χ2 test. BMI – body mass index, BP – blood pressure, AP – angina pectoris, STEMI – ST-segment elevation myocardial infarction, MINOCA – myocardial infarction with non-obstructive coronary arteries, NSP – null-stent patients.
Table III
Basic biochemical and haematological parameters of study participants
| Parameter | Control subjects (n = 84) | AP patients (n = 22) | STEMI patients (n = 93) | MINOCA patients (n = 6) | NSP (n = 7) | P-value |
|---|---|---|---|---|---|---|
| Total cholesterol# [mmol/l] | 5.70 ±0.82 | 4.47 ±1.12 | 5.52 ±1.22 | 6.83 ±0.61 | 5.41 ±0.84 | 0.080 |
| Triglycerides [mmol/l] | 1.25 (0.87–1.72) | 0.94 (0.74–1.70) | 1.72 (1.19–2.44)aa,b | 1.78 (0.64–2.11)aa | 2.31 (1.72–4.74) | < 0.001 |
| HDL-C# [mmol/l] | 1.50 ±0.37 | 1.30 ±0.212 | 1.14 ±0.395aa | 1.65 ±0.334aa | 0.88 ±0.198 | < 0.001 |
| LDL-C [mmol/l] | 3.27 (2.77–3.78) | 3.17 (2.77–3.78) | 3.33 (2.64–4.47) | 4.62 (3.71–5.08) | 2.95 (2.52–4.13)a | 0.009 |
| Creatine kinase [IU/l] | – | 53 (47–150) | 204 (100–487) | 178 (78–611) | 206 (90–319) | 0.367 |
| Troponin I [mg/l] | – | – | 0.14 (0.002–3.00) | 0.002 (0.002–2.5) | 0.002 (0.001–6.0) | 0.732 |
| Red blood cells# [× 1012/l] | 4.85 ±0.36 | 4.5 ±0.61 | 4.8 ±0.57 | 5.0 ±0.71 | 4.4 ±0.53 | 0.085 |
| Haemoglobin# [g/l] | 143 ±8.5 | 137 ±10.3 | 144 ±16.2b | 149 ±17.3c | 125 ±18.4 | < 0.001 |
| White blood cells# [× 109/l] | 6.8 ±1.64 | 9.3 ±2.65 | 12.2 ±4.29aa,bb | 9.4 ±2.30 | 15.6 ±9.25aa,b | 0.001 |
| Platelets [× 109/l] | 271 (230–313) | 213 (183–247) | 246 (204–303) | 222 (135–278) | 408 (349–494)b,c,d | < 0.001 |
| CRP [mg/l] | 0.50 (0.20–1.80) | 3.09 (0.85–6.89)a | 4.40 (2.05–9.68)aa,bb | 58.45 (2.98–133.57)aa,bb | 3.27 (1.20–5.83)a | < 0.001 |
a,b,c,d < 0.05 vs. control, AP, STEMI, MINOCA groups (a vs. control subjects, b vs. AP, c vs. STEMI);
aa,bb,cc,dd p < 0.001 vs. control, AP, STEMI, MINOCA groups respectively. Results are presented as medians with 25th and 75th percentile value or as mean ± standard deviation for normally distributed variables (#). ANOVA followed by Tukey’s post-hoc test for differences in subgroups was used for normally distributed parameters, Mann-Whitney and Kruskal-Wallis tests were used for parameters with non-normal distribution. AP – angina pectoris, STEMI – ST-segment elevation myocardial infarction, MINOCA – myocardial infarction with non-obstructive coronary arteries, NSP – null-stent patients.
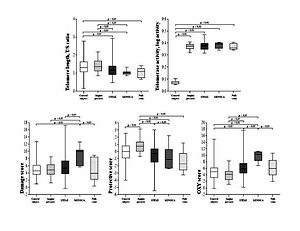
The telomere length, telomerase activity and oxidative-stress scores for the five main groups of patients and the control group in the study are presented in Figure 1. Significantly shorter telomeres were noted in all three groups compared to the control and AP groups. Among patient groups, the LTL were similar. Significantly higher values of telomerase activity were noted in all the subgroups of patients compared to the control group.
The MINOCA patients had the largest Damage score compared to other groups. The Protective score was the highest in the AP group compared to all the other groups. Values of OXY score was the highest in the MINOCA patients compared to the control group, AP and STEMI groups. In null-stent patients, Damage and OXY scores were significantly lower than in the MINOCA patients, while its Protective score was significantly lower as compared to the AP patients (Figure 1).
Table I summarises the results for STEMI patients obtained at different time points, and in different sample types (i.e. peripheral blood and thromboaspirates). LTL was, interestingly, significantly higher in the STEMI patients after the pPCI procedure compared to the same patients in the acute state. Six months after the acute event, following the pPCI procedure and appropriate subsequent cardiovascular therapy, LTL remained significantly lower compared to the control subjects, similar as it was at the time of the acute event. The telomerase activity showed significantly higher values in the acute state than in the control group. The telomerase activity values remained increased even at the follow-up 6 months after the acute event, compared to the controls and the same STEMI patients in the acute state. In fact, 6 months after the acute event STEMI patients’ telomerase activity was even more pronounced.
The Damage score was significantly higher in the STEMI patients’ acute state compared to the point after the pPCI intervention and 6 months after the acute event as well. On the other hand, the protective score was significantly lower after the pPCI intervention than in the acute state. The comprehensive OXY score was significantly lower after the pPCI procedure and at the 6-month follow-up compared to the STEMI patients in their acute state.
Regarding thromboaspirates, we noted longer telomeres in this patient sample than in peripheral blood leukocytes’ telomeres, but still shorter compared to LTL after the pPCI procedure. The telomerase activity in thromboaspirates was higher than in peripheral blood leukocytes of the control group. The protective score showed significantly higher values in thromboaspirates compared to the peripheral blood samples of the same patients, while the OXY score calculated for thromboaspirates was significantly lower compared to its OXY scores in blood samples (Table I).
Logarithmic transformation of non-parametrically distributed variables allowed multiple regression analysis (forward selection) to predict leukocyte telomere length and telomerase activity in the STEMI patients. Table IV presents the best model of parameters predicting LTL, consisting of telomerase activity, total cholesterol, HDL-C and LDL-C, able to significantly predict leukocyte telomere length values in the STEMI patients. The adjusted R2 value (0.564) indicates that the selected model explained almost 60% of variability in LTL.
Table IV
Models predicting telomere length in STEMI patients according to multiple linear regression analysis
Table V shows the most important selected ROC parameters of the model. Comparison of ROC curves showed that separate curves have comparable discriminatory capability towards STEMI status. The construction of a model consisting of these two independent parameters (leukocyte telomere length and telomerase activity) using logistic regression analysis showed that the new ROC curve had a good discriminatory ability to separate the STEMI patients from healthy persons (AUC = 0.711, cut-off (95% CI) = 0.544 (0.632–0.790)). Other tested models were not significant enough to be able to predict different subgroups.
Table V
Model for evaluation of telomere length clinical accuracy in STEMI patients
| AUC | Standard error | Asymptotic significance | Cut-off (95% CI) |
|---|---|---|---|
| 0.711 | 0.040 | < 0.001 | 0.544 (0.632–0.790) |
Furthermore, the STEMI patients were followed additionally after the pPCI interventions for the next 4 years. Re-infarction and death occurrence were followed as the long-term outcomes. A total of two lethal outcomes and one re-infarction were recorded. In order to understand the role LTL in CAD progression, we divided STEMI telomere length in tertiles. Both patients with a lethal outcome had values in the lowest tertile (LTL 0.933 and 0.975), while the result of the patient with re-infarction was on the border between the lowest and the middle tertile (LTL 1.074).
Discussion
In the current study we found significantly shorter leukocyte’s telomere length in all four CVD subgroups (i.e. AP, STEMI, MINOCA and the patients who experienced blood vessel rupture during the emergency post-infarction treatment). To our best knowledge, this was the first time that telomere length and telomerase activity were measured in MINOCA patients and in thrombus obtained during the pPCI intervention in combination with oxidative stress markers. The lack of difference in telomere length between cardiovascular entities suggests that the presence of the common pathological processes leading to CVD development is the main cause of telomere integrity impairment, independently of the further specific cardiovascular sub-cause. Margaritis et al. [30] reported LTL as a potential predictive parameter for CAD patients’ outcomes, but also as a possible predictive marker of circulating oxidative stress. However, the study used peripheral blood cells of AMI patients for LTL measurement at just one point combined with ex vivo studies on peripheral blood mononuclear cells for oxidative stress analysis. Along with that, we evaluated the balance between pro-oxidants and antioxidants through the several calculated comprehensive redox status scores in our patients. In the STEMI patients, the oxidative stress condition was evidenced through the high Damage score and exhausted antioxidative protection (Table I). This result suggests a possible influence of circulating pro-oxidants on LTL shortening, which could be one of the CAD triggers acting through the genomic instability amplification [31, 32]. On the other hand, increased BMI, diabetes mellitus and glucose intolerance (Table II) that were present as comorbidities in the CAD groups could certainly contribute to further LTL shortening as well as smoking habit, stress and anxiety that are often present in CAD patients, but they were not further evaluated in this study [25, 26, 27].
We also noted increased telomerase activity (as a key telomere length maintenance enzyme) in all four cardiovascular subgroups compared to the healthy persons. The exact mechanism of telomerase activity increasing in cardiovascular patients is unknown, but it is considered to be mediated by telomerase catalytic subunit (TERT) stimulation via formation of 8-oxo-7,8-dihydro-2′-deoxyguanine (8-oxoG) in telomeric DNA [13]. Similarly to telomeres, we did not observe a difference in telomerase activity among four different patient subgroups, which suggests that the enzyme might have overall cardiovascular protection capability.
Recent studies have associated statin therapy, common therapy in CAD patients, with higher telomerase activity [28, 29]. The percentage of patients using statin in their therapy is reported in Table II. A significantly higher percentage of AP patients reported statins in their common therapy compared to the STEMI subgroup, while none of the MINOCA nor NSP patients had them in their therapy. By telomerase activity we compared the patients on statin therapy with those that did not use them, and we did not detect an increase of the telomerase enzyme activity in the patients who used hypolipidemic therapy.
Focusing on the STEMI patients, severe oxidative stress has been found in our results. We also observed that pPCI intervention worsened oxidative stress in the STEMI patients. It is well known that reperfusion could increase free radical generation and thus complicate the redox situation in already damaged endothelium [33]. Coluzzi et al. [34], working on primary human fibroblasts, explained that oxidative stress damages telomeres probably by making modified bases (8-oxoG) and causing single strand breaks in telomere DNA capable of triggering genome instability, whereas Tian and collaborators reported a close connection between peripheral leukocyte telomere shortening and premature CAD [10]. The appearance of shorter LTL in atherosclerotic patients might be attributed to the increased leukocyte consumption during inflammatory processes and accelerated telomere loss per replication in enhanced oxidative stress conditions [35]. The reperfusion procedure, contrary to our expectations, did not cause LTL shortening. We found significant LTL elongation after the recanalization of occluded arteries (Table I). The exact cause of the mechanism of this process has not been elucidated, but we suppose that massive pro-oxidant generation after the recanalization intervention triggers survival compensatory mechanisms mirrored here in LTL telomere elongation to enable longer life of these important immune system players. A rising number of studies have reported that telomere shortening upon oxidative stress is likely to be tissue- and time-dependent. Dynamics of pro-oxidant change is probably faster than telomere length alteration [36], and this fact raises the question of the factors influencing our finding regarding telomere length change caused by pPCI. So, we hypothesized that forced blood flow, following recanalization of the occluded artery, brought unaffected leukocytes that could cause apparent leukocyte telomere lengthening, as seen in this study. Leukocytes are mobilized within hours of the AMI onset as part of a tissue injury healing response. On its way from bone marrow to the injured myocardium they must travel through the systemic circulation, and constitute part of the circulating blood cells [37, 38].
Our results showed increased telomerase activity in the STEMI patients at admission (Table I). The effects of oxidative stress on telomerase are still not well understood. While some studies [12, 13] suggested that reactive oxygen species promote telomerase activity, the Aeby research group [39] proposed repression of telomerase activity upon oxidative stress conditions. We confirmed that telomerase activity is upregulated with impairment of oxidative stress, which happened after occluded artery recanalization. Moreover, it remained highly active even 6 months after the acute event. This finding proves that still severe prooxidant levels promote telomerase activity most likely via destabilisation of G-quadruplex structures in the DNA due to formation of 8-oxoG in telomeric DNA, as suggested by Fouquerel et al. [14]. In concordance with still short telomeres and a high Damage score, these results confirm the direct relation between telomeres and STEMI pathology, as well as suggesting that a period longer than 6 months is needed for the whole recovery process after the AMI.
Silvain et al. reported a highly complex composition of the thrombus [40]. Our analysis of telomere length and telomerase activity directly in thrombus material showed results similar to the acute state, suggesting that once CAD has appeared it could impair the telomere structure, making it incapable of performing its physiological function and modifying telomerase by continuously accelerating its activity rate, most likely using the same mechanisms of telomerase stimulation by 8-oxoG formation as previously mentioned. Narducci et al. performed a similar experiment and also observed high telomerase activity in polymorphonuclear neutrophils of the coronary plaques obtained from angioplasty washing medium [41]. Redox status related scores in our study indicate the state of severe oxidative stress that is probably related to the telomere structure change and telomerase activity performance. To the best of our knowledge, this is the first time that a relation has been reported between the telomere-telomerase system and oxidative stress status of the whole atherosclerotic thrombus obtained during a pPCI intervention.
A moderate correlation was reported between leukocyte telomere length and triglyceride (negative) and HDL-C levels (positive) [42], and negative with hypercholesterolaemia in young individuals, as well [43]. We noted a different association in our research. Namely, we found that telomere length as a parameter could be strongly predicted by telomerase activity and total cholesterol, HDL-C and LDL-C (Table I). Short leukocyte telomeres might be connected to lipid status through various molecular mechanisms, such as increased inflammation and oxidative damage, conditions that have been already associated with the telomere attrition rate [44], but the precise mechanism still remains unclear. These results also suggest the potential role of LTL as a marker for CAD progression.
Our results are in accordance with the literature data by showing that LTL might be associated with CAD patients’ outcomes, as also suggested by other authors [25] who reported LTL as a predictor of post-AMI cardiovascular outcomes regardless of age. Indeed, the two deceased patients and the patient who experienced re-infarction in our study according to 4-year follow-up data had the LTL in the lowest tertile group. Still, further research is needed to understand this crosstalk better.
The results of the present study should be interpreted carefully. Our study included only Serbian subjects, and it should not be extended to other groups without additional confirmatory studies being conducted on other populations. The measurement of leukocyte telomere length, telomerase activity and oxidative stress parameters may not have clinical utility at the moment, but a great number of similar studies might emphasise the importance of finding novel, less complex analytical methods for those parameters that would qualify them as relevant and applicable for clinical use.
The findings of this study have to be considered in light of certain limitations. The first is the limited sample size. The restrictive inclusion criteria cause a small size of samples included in the study, especially in the MINOCA patient group, which has the prevalence of 5–6% [5] of all acute myocardial infarctions. The second limitation concerns the risk factors such as smoking habit, stress and anxiety that are often present in CAD patients. The stress and anxiety evaluations require specialised questionnaires, and they were not included in our study. Regarding the number of smokers, there is a discrepancy between the patients and the control group, with more smokers being in the patient group. To eliminate its effect, the control group should consist of only healthy smokers, which this study could not provide. Stress and anxiety could also cause imbalance in the obtained results. Their impact should be evaluated using more complex and specialised questionnaires that were unfortunately not included in this study.
In conclusion, our study suggests a close connection between the telomere-telomerase system and oxidative stress as one of the significant factors in CAD development. Leukocyte telomere length and telomerase activity have the ability to differentiate CAD patients from healthy persons.
The data obtained in this hypothesis-generating study suggest that shorter telomeres in these patients could be connected with poor patients’ outcome. However, due to the small sample size and confounding factors mentioned above, a more comprehensive study with an adequate number of patients would probably unveil this tight crosstalk between the telomere-telomerase system, oxidative stress and CAD.