Introduction
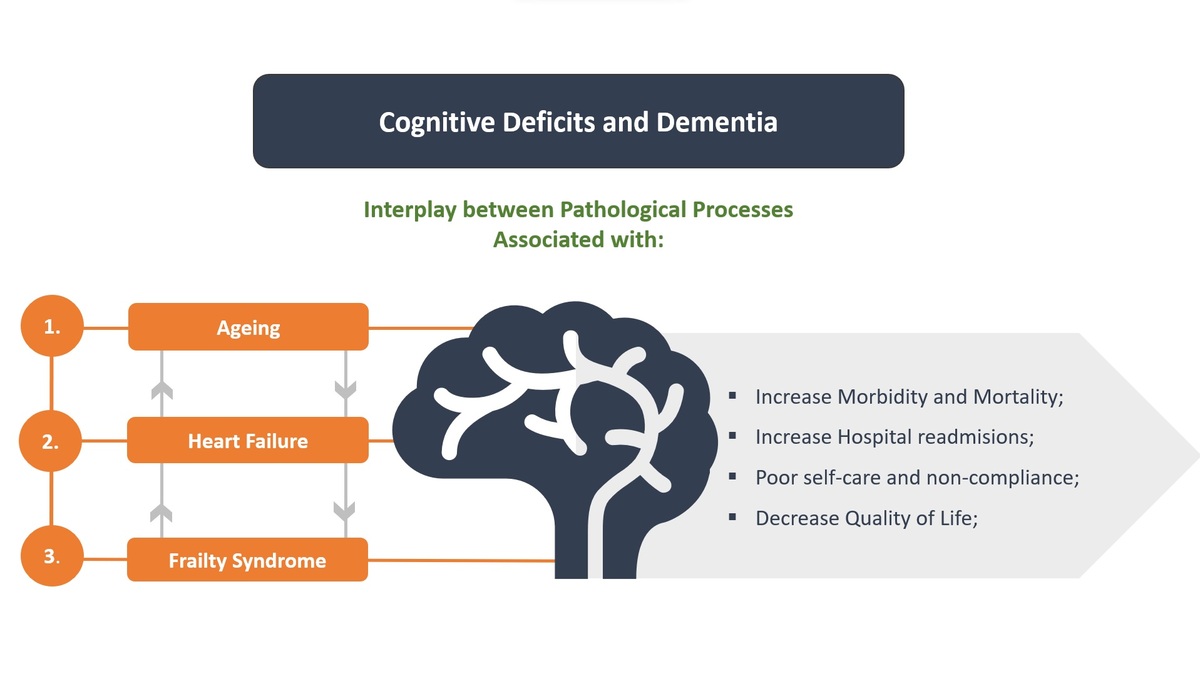
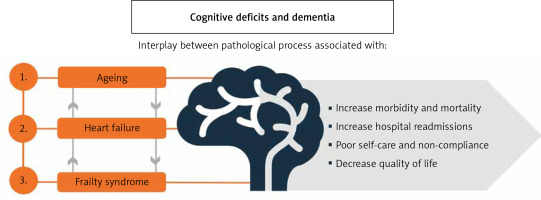
Ageing is reported as the most important risk factor for cognitive impairment and dementia [1]. However, there is also evidence that many age-associated processes that lead to frailty in older people are also responsible for brain ageing and consequent cognitive decline [2]. Frailty is a complex clinical syndrome characterized by decreased physiological reserve and increased vulnerability to stressors [3]. There is evidence that frailty syndrome is reversible through appropriate interventions. From a clinical perspective, frailty has primarily been considered a decline in physical function (the so-called physical frailty). Recently, however, the decline in neuropsychiatric status, including cognitive impairments and depression, has been shown to be associated with frailty syndrome [3] (Figure 1).
In the heart failure (HF) population, existing cognitive impairment is linked to frailty, and from the ageing perspective, frailty represents a potential risk factor for developing cognitive decline and prevalence of dementia in patients with HF. Several recent epidemiological studies have provided evidence that frailty can be an early indicator of cognitive decline, even after considering baseline cognitive function. Furthermore, recent research findings have established the link between frailty and the cognitive domains of executive function, attention, and processing speed. However, more work on the value of frailty measures in identifying those at risk of preventable cognitive decline in cardiovascular populations is needed [3].
Frailty syndrome and cognitive impairment
Interest in the mechanisms underlying the link between frailty in ‘pre-dementia’ cognitive states and frailty in dementia has escalated over the last 2 decades. Many epidemiological studies have explored these associations and disentangled the concepts of frailty and cognition to better understand their relationship [2]. Recent reviews on frailty report that physical function, gait speed, and cognition are most commonly included in a definition of frailty, but only 50% of the definitions included a cognition component [4]. Other analyses revealed that cognition does not correlate strongly with the frailty components of physical activity, mobility, energy, strength, and mood, indicating that cognition may not be part of the frailty syndrome [5, 6]. Furthermore, a recent study of Alzheimer’s dementia (AD) found that 22% of patients had no indications of frailty [7]. Taken together, these findings suggest that frailty syndrome and cognitive decline are related but distinct concepts that frequently cooccur [2].
Many cross-sectional studies have provided evidence of the association between cognitive impairment and frailty [2]. For example, 22% of frail participants enrolled in the Three City Study demonstrated cognitive impairment (defined as being in the lowest quartile of both the Isaac’s Set Test and the Mini Mental State Examination (MMSE)) compared to only 12% in the pre-frail population and 10% in the non-frail population [8]. The association between cognitive function and frailty remains consistent regardless of the method used to measure frailty (e.g. Fried’s biological model of frailty vs. Rockwood’s cumulative burden index) [9]. A study of home care recipients (23,952 participants) revealed that 40% of individuals classified as most frail based on the Rockwood’s frailty index had a diagnosis of dementia compared to 11% of those classified as least frail [10]. In another study, among 109 patients diagnosed with dementia, 50% were frail, 28% were pre-frail only, and 22% had no indications of frailty [7, 11]. These studies provide evidence that frailty and dementia co-occur, but they are unable to provide evidence of a causal association because they are cross-sectional. There also is evidence from longitudinal studies that greater frailty predicts future cognitive decline and dementia [9, 12–17]. An analysis of data from the Three City Study demonstrated that frailty was associated with incident vascular dementia but not with AD or other dementias [14]. The reciprocal relationship, that cognitive impairment indicates the future risk of frailty, has also been reported in epidemiological studies based on populations of community-dwelling older adults [18, 19]. These findings suggest that frailty may be either a cause of cognitive impairment or a consequence of cognitive impairment.
Recent studies indicate that frailty is associated with a more rapid rate of decline in specific domains of cognition. For instance, the Brazilian FIBRA study used the MMSE to evaluate cognitive function and showed that frail participants demonstrated greater impairment in orientation, commands, immediate memory, verbal fluency, and clock drawing tasks, but not on delayed memory [20, 21]. Findings of the Rush Memory and Aging Study showed that frailty was associated with measures of global cognition and perceptual speed but not with episodic memory, semantic memory, working memory, or visuospatial ability [15]. A study of community-living older adults provided evidence that frailty was predictive of performance in executive function and processing speed but not global cognition, episodic memory, working memory, or verbal abstract reasoning [22]. Similar findings were reported from a study of inpatients in a geriatric rehabilitation ward [23]. After adjusting for age and depression, higher frailty measured by a cumulative burden of illness scale was associated with executive function, reasoning, dual-attentional processing, verbal fluency, and processing speed, but not with memory [23]. Importantly, across studies, memory does not appear to be strongly related to frailty [21, 23]. Although frail participants had poorer memory scores in several studies, the scores failed to reach the threshold for statistical significance [15, 20, 21, 24]. The emerging pattern of cognitive decline from these studies suggests that executive function and attention are most strongly associated with the syndrome of frailty. This may be partly explained by cardiovascular factors, because both frailty and executive function impairment have been associated with cardiovascular disease [24].
Cognitive impairment in HF
As is true of frailty, cognitive impairment is particularly prevalent in HF. Patients with HF have a higher risk for cognitive impairments than individuals without HF, after controlling for age, sex, and comorbidities [25]. Physical frailty, social frailty, and cognitive dysfunction are prevalent among hospitalized elderly patients with HF and considerably overlap each other. The overlap becomes more pronounced as age increases, and a greater number of frailty domains is associated with a worse prognosis [26].
Clinical studies indicate that the incidence of cognitive impairment in patients diagnosed with HF ranged from 23% to 80%, depending on the type of frailty measures used and the characteristics of the HF sample [27, 28]. As opposed to patients without HF, patients of similar age with HF exhibited deficits in the domains of memory, executive function, attention, and psychomotor speed [29]. In contrast, language and visuospatial ability are less affected in patients with HF, although only a few studies evaluated these cognitive abilities in patients with HF. Interestingly, Athilingam et al. showed that the pattern of impaired cognitive domains varied between patients with HF with reduced ejection fraction and patients with HF with preserved ejection fraction [30]. The above findings might be associated with the pathophysiology of cognitive impairment in patients with HF, including the syndrome of frailty. Although there is significant epidemiological evidence linking phenomena of cognitive decline and frailty, little work has directly explored mechanisms underlying this association in a population with HF. Therefore, further investigations on the pathophysiological interactions between HF, physical frailty, and cognition are needed to identify strategies to treat and prevent cognitive impairment in elderly patients with HF.
As outlined above, several recent epidemiological studies have established the association between frailty and deficits in the domains of executive function, attention, and processing speed. Furthermore, these studies have provided evidence that frailty can be an early indicator for cognitive decline in the CVD population even after considering cardiovascular factors. Findings of previous research indicate that slow gait speed predicts the future onset of dementia and mild cognitive impairment (MCI) [31, 32]. Furthermore, Buracchio et al. indicated that gait speed decline might occur as early as 12 years before the onset of MCI [33]. Because changes in gait may appear earlier than cognitive changes, assessment of gait speed should be incorporated in risk assessment [34]. The repeated measures of gait speed could provide an early screening tool for increased dementia risk. However, more work on the potential ability of frailty to identify those at risk of preventable cognitive decline is needed in cardiovascular populations.
The prevalence of frailty syndrome and HF is increasing [35]. The robust increase of frailty and HF in the elderly population is concerning due to the associated high mortality rates and economic costs [36, 37]. Clinicians should focus more attention on geriatric conditions, including multiple morbidities, polypharmacy, disability, malnutrition, frailty, and cognitive impairment, because each of these conditions significantly affects the course of HF, its management, and its prognosis in the elderly [3, 27].
Cardiovascular risk factors and HF
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality [38]. Within the ageing population, the prognostic determinants of morbidity and mortality due to CVD include frailty, health status, disability, and cognition [39, 40]. In particular, frailty is a powerful predictor of poor clinical outcomes and mortality in cardiovascular disease [39, 40]. Frail patients with CVD are more likely to develop adverse outcomes than their non-frail counterparts, particularly those undergoing invasive procedures or HF [41]. Frail persons have twice the risk of HF and increased rates of mortality (16.9% vs. 4.8%) when compared with the non-frail [42]. Frailty syndrome is present in 25% to 60% of patients with CVD, depending on the operational definition of frailty (i.e. specific frailty measure) and the specific patient population (i.e. HF vs. acute coronary syndrome) [43]. Frailty and CVD share common biological pathways. In fact, CVD may accelerate the development of frailty. There exists a bidirectional association between frailty and CVD, where frailty may lead to CVD and CVD may lead to frailty.
More severe HF combined with frailty may lead to an increased risk of comorbidities (hypertension, chronic kidney disease, diabetes, coronary heart disease, stroke, chronic lower respiratory tract disease), which in turn worsens cardiovascular outcomes (including extended time of hospitalization, disability, increase rehospitalizations, and mortality) [40]. Furthermore, the literature also points out a relationship between New York Heart Association (NYHA) classification (a measure of HF severity) and cognitive impairment. The current literature shows that patients with severe HF, defined by NYHA symptom class III to IV, have more cognitive impairment than patients with class I and II [44–47].
Frailty and comorbidity are clinical manifestations of 2 distinct age-related processes resulting from an accumulation of pathological processes and diminished functional reserve. Comorbid illnesses are prevalent in the older population. Wolff reported that 82% of the 1999 Medicare beneficiaries aged ≥ 65 years had one or more chronic conditions and 65% had multiple chronic conditions [48]. Also, Braunstein et al. demonstrated that 40% of the elderly HF population had ≥ 5 comorbidities, 70% had ≥ 3 comorbidities, and only 4% had no comorbidities at all [49]. There is growing evidence indicating that comorbidities contribute significantly to worse outcomes among those with HF than in those without HF [50, 51]. The published cohort studies of older adults show that chronic diseases (hypertension, chronic kidney disease, diabetes, coronary heart disease, stroke, chronic lower respiratory tract disease) are associated with frailty [52].
The European Society of Cardiology guidelines divide HF into 3 main subtypes based on left ventricular ejection fraction (LVEF): (a) HF with preserved ejection fraction (HFpEF); (b) HF with mildly reduced ejection fraction (HFmrEF); and (c) HF with reduced ejection fraction (HFrEF) [53]. Clinical characteristics and comorbidities differ in HFpEF and HFrEF [54]. For example, older age, atrial fibrillation, and pulmonary hypertension are more common in HFpEF than in HFrEF [55]. HF subtypes (HFpEF vs. HFrEF) may mediate the relationship between comorbidities and HF outcomes. Recently, Chen et al. reported that mortality and hospitalization rates are better in HFpEF than in HFrEF [56]. It was also shown that cardiac comorbidities are more likely in HFrEF, but non-cardiac comorbidities are more probable in HFpEF [56]. The clinical characteristics of HFmrEF patients are more similar to the HFrEF group than to the HFpEF group. These are more often male patients, younger, and with more frequent coronary artery disease. Atrial fibrillation and extracardiac comorbidities are less common in this group. Mortality is lower than in HFrEF patients [56].
Among HF subtypes, frailty syndrome is more prevalent in patients with HFpEF (60–90%) than HFrEF [57]. This finding may be explained by older age and higher comorbidity burden among HFpEF versus HFrEF patients [57]. The greater frailty was associated with a higher risk of cardiovascular outcomes and mortality, and patients with HFpEF and a high Frailty Index (FI) versus a low FI (> 0.5 vs. < 0.3) had a markedly higher risk of HF hospitalization and all-cause mortality [57]. However, another report suggested no differences in mortality between HFrEF (33%) and HFpEF (29%) [58]. A systematic assessment performed periodically to identify prefrailty may be the best strategy for preventing further functional loss and disability in persons with HF. Physical frailty and cognitive impairments are manifestations of complex aging syndromes with a pathology that often affects more than one physiological system [59]. Individuals with HF ideally should be evaluated annually for physical and cognitive function because a decline has consistently been shown to be a strong predictor of adverse health outcomes, disability, and death.
Cognitive impairments (CI) are frequently observed in the population of patients with a diagnosis of HF. The combination of CI and HF is associated with increased mortality, rehospitalization, and poor quality of life. Both HFrEF and HFpEF are associated with impaired cognitive function and may, at times, manifest as delirium in hospitalized patients or mild cognitive impairment in otherwise stable HF persons [60]. HF negatively affects cognitive function in most domains, but memory and executive functions are most often affected. It should be mentioned that deficits in these domains can significantly reduce the patient’s ability to perform basic self-care behaviours [61, 62]. In fact, 25% of patients with HF have cognitive decline that is severe enough to make it difficult for the individual to perform activities of daily living, engage in social activities, or complete job responsibilities [63]. Deficits in activities of daily living were considered conceptually distinct from the original Fried frailty phenotype, with frailty often serving as a marker of risk for disability in functionally independent, community-dwelling elders [64, 65]. However, assessment of activities of daily living has been successfully incorporated into several frailty phenotype models that are particularly relevant in older and sicker patients with cardiovascular disease.
Several studies have demonstrated impairments of one or more cognitive domains in people with HFrEF, but there are few data on the domains that are affected among people with HFpEF [66]. There is evidence that the domains of attention and executive functioning are most often affected in HFrEF [67]. Similarly, Gottesman et al. demonstrated that early stages of diastolic dysfunction were associated with worse cognitive function in the same domains [68]. Another study proved that the memory domain is similarly impaired in participants with HFrEF and HFpEF [30]. Warraich et al. showed that patients ≥ 60 years of age hospitalized with ADHF have a broad, marked impairment of physical function and a high rate of frailty and impairment of cognitive functions. This study showed that these disorders are similar between HFpEF and HFrEF [69].
Mental health and cognitive decline in frail patients with HF
Mood disorders, such as depression and anxiety, are prevalent in both heart disease and HF [70, 71]. Depression and anxiety also often coexist with frailty syndrome [70, 71]. In fact, mood disorders such as depression and anxiety have both been found to be a risk factor for and a consequence of frailty [72, 73]. Depression is known to affect cognitive function [74]. These results suggest that there may be a link between frailty, depressive symptoms, and cognitive decline in the cardiovascular population. In depression, the affected cognitive domains include attention, executive function, memory, and processing speed [75, 76]. These cognitive impairments tend to persist even during the remission of depressive symptoms. In prevalence terms, of those with major depressive disorder 85–94% report having cognitive difficulties during depressive episodes and 39–44% report having cognitive impairments during remissions [77]. In some cases of major depressive disorder, the loss of higher cognitive functions dominates the clinical picture and creates a picture of “pseudodementia” [78, 79]. In clinical practice, it is challenging to distinguish between depression-related cognitive impairment that can be reversed and cognitive impairment due to other causes (degenerative or vascular) destined to advance. A study of 2615 participants (mean age: 70.2 years, 51.1% female) demonstrated a curvilinear relationship between cognitive function and anxiety, indicating that cognitive function was better among individuals with mild anxiety symptoms compared to people with severe anxiety symptoms [80].
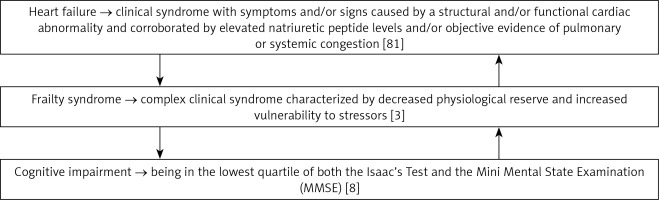
Figure 2 provides definitions of the interplay between HF, frailty, and cognitive impairment [3, 8, 81].
Self-care and adherence behaviour in a population of frail patients with HF
Among the critical factors for maintaining health, minimizing disease complications, and improving quality of life in patients with cardiovascular disease are behaviours relevant to self-care and adherence [82, 83]. Self-care is defined as a “naturalistic decision-making process in which persons engage to maintain health and manage acute and chronic illness” [83]. This implies that patients have the psychological ability and cognitive resources to undertake activities directed at self-care. The healthcare system is built on the assumption that patients comply with healthcare providers’ recommendations, but not all patients have the capacity to do so [83]. It has also been shown that several cultural and individual factors influence self-care behaviours [82, 83]. Studies show that patients usually have the necessary knowledge, skills, confidence, and motivation to engage in self-care; however, most need assistance in understanding how to actively process, understand, and evaluate the required self-care behaviours. As indicated earlier, cognitive deficits among patients with CVD are associated with impairments in memory, learning, attention, and executive function [46, 67, 84, 85]. Moreover, depression can lead to poor self-care and non-compliance with healthcare guidelines. Thus, these cognitive and emotional-motivation problems of HF patients can lead to patients’ inability to engage in effective self-care behaviour and negatively influence outcomes.
Summary and future directions
In previous studies, cognitive training led to small improvements in cognitive function, as well as positive effects on engagement in HF self-care and functional outcomes such as instrumental activities of daily living (e.g. medication taking), which may result in reduced healthcare costs [86, 87]. Cognitive training may be conducted by nurses in the absence of physicians. Nurses are indispensable to the care of patients diagnosed with HF and their families. A growing body of scientific knowledge explores models and organization of HF care, facilitation of patients’ participation in their self-care and interventions to improve patient outcomes. However, additional research of high quality and rigorous methodology is needed to advance nursing science and improve the lives of patients with HF and their families [88].
In everyday clinical practice, identifying frailty may enable better risk stratification of patients with HF, optimize individualized care plans for patients, minimize negative outcomes, and reduce health care costs [89]. The assessment of frailty should be part of a comprehensive assessment of HF patients and one of the first steps for accurate risk stratification and a tailored therapeutic plan. Table I summarizes selected solutions affecting cognitive impairment and frailty in HF [90–92].
Table I
Interventions that can positively affect cognitive impairment and frailty in HF