Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / CLINICAL RESEARCH
The effectiveness of sulforaphane in radiosensitizing breast cancer cells
1
Oncology Research Laboratory, Oncology Institute, Faculty of Medicine, Medical Academy Lithuanian University of Health Sciences, Kaunas, Lithuania
2
Department of Genetics and Molecular Medicine, Faculty of Medicine, Medical Academy Lithuanian University of Health Sciences, Kaunas, Lithuania
3
Department of Oncology and Hematology, Hospital of Lithuanian University of Health Sciences, Kaunas, Lithuania
4
Physics Department, Kaunas University of Technology, Kaunas, Lithuania
5
Department of Radiology, Faculty of Medicine, Medical Academy Lithuanian University of Health Sciences, Kaunas, Lithuania
6
Oncology Institute, Faculty of Medicine, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania
Submission date: 2025-07-02
Final revision date: 2025-07-17
Acceptance date: 2025-07-17
Online publication date: 2025-07-26
Corresponding author
Danguole Laukaitiene
Institute of Oncology Oncology Research Laboratory Lithuanian University of Health Sciences Mickevičiaus str. 9 LT 44307 Kaunas Lithuania Phone: +37067746494
Institute of Oncology Oncology Research Laboratory Lithuanian University of Health Sciences Mickevičiaus str. 9 LT 44307 Kaunas Lithuania Phone: +37067746494
KEYWORDS
TOPICS
ABSTRACT
Introduction:
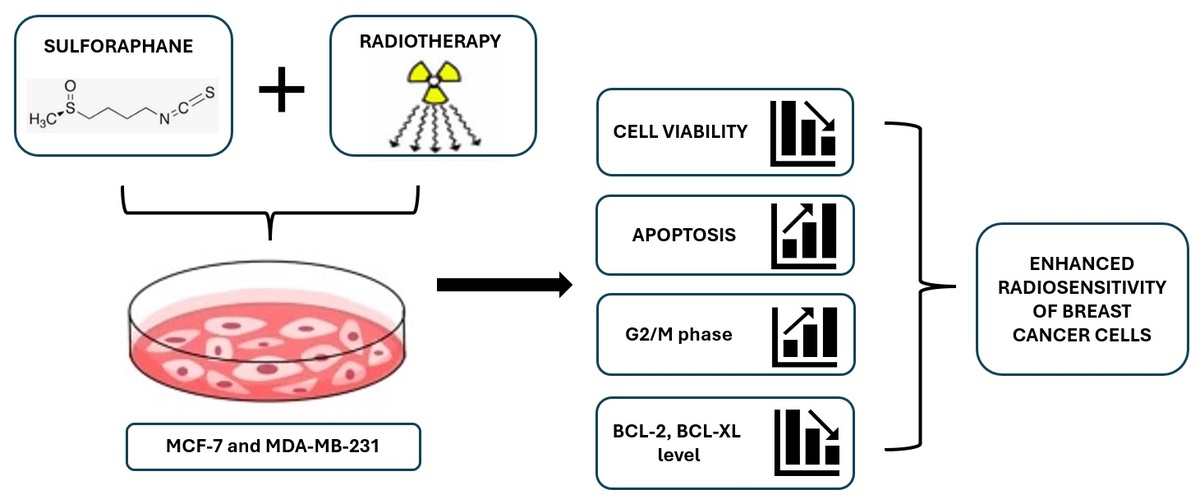
Radiotherapy is a vital therapeutic option in the treatment of breast cancer nowadays. However, a major obstacle to the full effectiveness of radiation therapy is still the radioresistance of cancer cells. Various studies have proven sulforaphane’s (SFN) beneficial effects against cancer and its possible utilization as a radiosensitizer in radiotherapy. This study aimed to investigate whether SFN has a radiosensitizing effect on breast cancer cells.
Material and methods:
The anticancer efficiency of SFN and radiosensitizing effect in MCF-7 and MDA-MB-231 cell lines were assessed by the MTT assay. Using a flow cytometric assay, the apoptosis level and changes in the cell cycle were measured. RT-qPCR and Western blot analysis were used to determine the expression and protein levels of the BCL-2 and BCL-XL genes.
Results:
According to our results, SFN reduced the viability of cells, and combining SFN with radiation therapy (IR) caused much greater anticancer effects on cells. SFN + IR was shown to enhance the number of cells in the G2/M phase and the percentage of cells undergoing apoptosis. SFN reduced the expression of the apoptosis-related genes BCL-2 and BCL-XL. Consistent with these data, Western blot analysis revealed that BCL-2 and BCL-XL protein levels were decreased in tested cells. As a result of the combination treatment, downregulation of the BCL-2 protein was observed only in MDA-MB-231 cells.
Conclusions:
These results indicate that SFN acts as a radiosensitizer by enhancing apoptotic cell death and reducing anti-apoptotic gene expression in breast cancer cells.
Radiotherapy is a vital therapeutic option in the treatment of breast cancer nowadays. However, a major obstacle to the full effectiveness of radiation therapy is still the radioresistance of cancer cells. Various studies have proven sulforaphane’s (SFN) beneficial effects against cancer and its possible utilization as a radiosensitizer in radiotherapy. This study aimed to investigate whether SFN has a radiosensitizing effect on breast cancer cells.
Material and methods:
The anticancer efficiency of SFN and radiosensitizing effect in MCF-7 and MDA-MB-231 cell lines were assessed by the MTT assay. Using a flow cytometric assay, the apoptosis level and changes in the cell cycle were measured. RT-qPCR and Western blot analysis were used to determine the expression and protein levels of the BCL-2 and BCL-XL genes.
Results:
According to our results, SFN reduced the viability of cells, and combining SFN with radiation therapy (IR) caused much greater anticancer effects on cells. SFN + IR was shown to enhance the number of cells in the G2/M phase and the percentage of cells undergoing apoptosis. SFN reduced the expression of the apoptosis-related genes BCL-2 and BCL-XL. Consistent with these data, Western blot analysis revealed that BCL-2 and BCL-XL protein levels were decreased in tested cells. As a result of the combination treatment, downregulation of the BCL-2 protein was observed only in MDA-MB-231 cells.
Conclusions:
These results indicate that SFN acts as a radiosensitizer by enhancing apoptotic cell death and reducing anti-apoptotic gene expression in breast cancer cells.
REFERENCES (45)
1.
Hussain A, Mohsin J, Prabhu SA, et al. Sulforaphane inhibits growth of human breast cancer cells and augments the therapeutic index of the chemotherapeutic drug, gemcitabine. Asian Pac J Cancer Prev 2013; 14: 5855-60.
2.
Smolarz B, Nowak AZ, Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (review of literature). Cancers (Basel) 2022; 14: 2569.
3.
Pagliari F, Jansen J, Knoll J, Hanley R, Seco J, Tirinato L. Cancer radioresistance is characterized by a differential lipid droplet content along the cell cycle. Cell Div 2024; 19: 14.
4.
Chougule A. Status of cancer treatment by radiotherapy and requirement of radiation oncology medical physicists in Asia Oceania federation of organizations for medical physics region. J Cancer Res Ther 2023; 19: 567-72.
5.
Ko YS, Jin H, Lee JS, et al. Radioresistant breast cancer cells exhibit increased resistance to chemotherapy and enhanced invasive properties due to cancer stem cells. Oncol Rep 2018; 40: 3752-62.
6.
Wu Y, Song Y, Wang R, Wang T. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer 2023; 22: 96.
7.
Cao X, Wen P, Fu Y, et al. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell Signal 2019; 62: 109337.
8.
Darvish L, Bahreyni Toossi MT, Azimian H, et al. The role of microRNA-induced apoptosis in diverse radioresistant cancers. Cell Signal 2023; 104: 110580.
9.
Abdul Rahman SF, Muniandy K, Soo YK, et al. Co-inhibition of BCL-XL and MCL-1 with selective BCL-2 family inhibitors enhances cytotoxicity of cervical cancer cell lines. Biochem Biophys Rep 2020; 22: 100756.
10.
Hara T, Omura-Minamisawa M, Chao C, Nakagami Y, Ito M, Inoue T. Bcl-2 inhibitors potentiate the cytotoxic effects of radiation in bcl-2 overexpressing radioresistant tumor cells. Int J Radiat Oncol Biol Phys 2005; 61: 517-28.
11.
Tiwari P, Mishra KP. Flavonoids sensitize tumor cells to radiation: molecular mechanisms and relevance to cancer radiotherapy. Int J Radiat Biol 2020; 96: 360-9.
12.
Asif Ali M, Khan N, Kaleem N, et al. Anticancer properties of sulforaphane: current insights at the molecular level. Front Oncol 2023; 13: 1168321.
13.
Zhou T, Zhou M, Tong C, Zhuo M. Cauliflower bioactive compound sulforaphane inhibits breast cancer development by suppressing NF-B /MMP-9 signaling pathway expression. Cell Mol Biol 2022; 68: 134-43.
14.
Kotowski U, Heiduschka G, Brunner M, et al. Radiosensitization of head and neck cancer cells by the phytochemical agent sulforaphane. Strahlenther Onkol 2011; 187: 575-80.
15.
Wang S, Wang Y, Liu X, Yang Y, Wu S, Liu Y. SFN enhanced the radiosensitivity of cervical cancer cells via activating LATS2 and blocking Rad51/MDC1 recruitment to DNA damage site. Cancers (Basel) 2022; 14: 1872.
16.
Ren K, Li Z, Li Y, Zhang W, Han X. Sulforaphene enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-B pathway. J Biochem Mol Toxicol 2017; 31: 8. doi: 10.1002/jbt.21917.
17.
Liu M, Yao X, Li W, et al. Nrf2 sensitizes prostate cancer cells to radiation via decreasing basal ROS levels. Biofactors 2015; 41: 52-7.
18.
Yu D, Sekine-Suzuki E, Xue L, Fujimori A, Kubota N, Okayasu R. Chemopreventive agent sulforaphane enhances radiosensitivity in human tumor cells. Int J Cancer 2009; 125: 1205-11.
19.
Gamet-Payrastre L, Li P, Lumeau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 2000; 60: 1426-33.
20.
Pogorzelska A, Świtalska M, Wietrzyk J, et al. Antitumor and antimetastatic effects of dietary sulforaphane in a triple-negative breast cancer models. Sci Rep 2024; 14: 16016.
21.
Naumann P, Liermann J, Fortunato F, et al. Sulforaphane enhances irradiation effects in terms of perturbed cell cycle progression and increased DNA damage in pancreatic cancer cells. PLoS One 2017; 12: e0180940.
22.
Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther 2007; 6: 1013-21.
23.
Beaver JA, Gustin JP, Yi KH, et al. PIK3CA and AKT1 mutations have distinct effects on sensitivity to targeted pathway inhibitors in an isogenic luminal breast cancer model system. Clin Cancer Res 2013; 19: 5413-22.
24.
Mahmoud A, Casciati A, Bakar ZA, Hamzah H, Ahmad TAT, Noor MHM. The detection of DNA damage response in MCF7 and MDA-MB-231 breast cancer cell lines after X-ray exposure. Genome Integr 2023; 14: e20220001.
25.
Wagner K. Know thy cells: commonly used triple-negative human breast cancer cell lines carry mutations in RAS and effectors. Breast Cancer Res 2022; 24: 44.
26.
Langlands FE, Horgan K, Dodwell DD, Smith L. Breast cancer subtypes: response to radiotherapy and potential radiosensitisation. Br J Radiol 2013; 86: 20120601.
27.
Licznerska B, Szaefer H, Matuszak I, Murias M, Baer-Dubowska W. Modulating potential of L-sulforaphane in the expression of cytochrome p450 to identify potential targets for breast cancer chemoprevention and therapy using breast cell lines. Phytother Res 2015; 29: 93-9.
28.
Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One 2010; 5: e11457.
29.
Pawlik A, Wiczk A, Kaczyńska A, Antosiewicz J, Herman-Antosiewicz A. Sulforaphane inhibits growth of phenotypically different breast cancer cells. Eur J Nutr 2013; 52: 1949-58.
30.
Ramirez MC, Singletary K. Regulation of estrogen receptor alpha expression in human breast cancer cells by sulforaphane. J Nutr Biochem 2009; 20: 195-201.
31.
Sakao K, Singh SV. D,L-sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc. J Cell Biochem 2012; 113: 599-610.
32.
Choi S, Singh SV. Bax and bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res 2005; 65: 2035-43.
33.
Hać A, Brokowska J, Rintz E, Bartkowski M, Węgrzyn G, Herman-Antosiewicz A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur J Nutr 2020; 59: 1421-32.
34.
Quiet CA, Weichselbaum RR, Grdina DJ. Variation in radiation sensitivity during the cell cycle of two human squamous cell carcinomas. Int J Radiat Oncol Biol Phys 1991; 20: 733-8.
35.
Zhao Y, Jiang W, Li B, et al. Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int Immunopharmacol 2011; 111: 2039-46.
36.
Rosser CJ, Reyes AO, Vakar-Lopez F, et al. Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int J Radiat Oncol Biol Phys 2003; 56: 1-6.
37.
Park SY, Kim GY, Bae S, Yoo YH, Choi YH. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase-3. Oncol Rep 2007; 18: 181-7.
38.
Kim S, Park H, Moon D. Sulforaphane sensitizes human breast cancer cells to paclitaxel-induced apoptosis by downregulating the NF-B signaling pathway. Oncol Lett 2017; 13: 4427-32.
39.
Amer MA, Mohamed TR, Rahman RAA, Shalaby MA, Badr A. Improvement of sulforaphane production in hairy root cultures of broccoli (brassica oleracea L. var. italica) by eliciting myrosinase gene expression and its effect on breast cancer cells. Plant Cell Tiss Organ Cult 2024; 158: 1.
40.
Park MJ, Kim YH. Anti-cancer effect of sulforaphane in human pancreatic cancer cells mia PaCa-2. Cancer Rep (Hoboken) 2024; 7: e70074.
41.
Habib TN, Altonsy MO, Ghanem SA, Salama MS, Hosny MA. Optimizing combination therapy in prostate cancer: Mechanistic insights into the synergistic effects of paclitaxel and sulforaphane-induced apoptosis. BMC Mol Cell Biol 2024; 25: 5.
42.
Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res 2008; 14: 6855-66.
43.
Zhang Z, Li C, Shang L, et al. Sulforaphane induces apoptosis and inhibits invasion in U251MG glioblastoma cells. Springerplus 2016; 5: 235.
44.
Yasunaga A, Ono M, Takeshima M, Nakano S. Sulforaphane suppresses the growth of EGFR overexpressing MDA MB 468 triple negative breast cancer cells in vivo and in vitro. Int J Funct Nutr 2022; 3: 2.
45.
Campbell KJ, Tait SWG. Targeting BCL-2 regulated apoptosis in cancer. Open Biol 2018; 8: 180002.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.