Introduction
Menopause is a natural biological milestone that most women will encounter as they progress through midlife, significantly affecting their health, well-being, and overall quality of life [1]. This stage signifies the permanent end of menstrual cycles, which is clinically confirmed after a woman has gone 12 months without menstruating, a process that typically takes place between the ages of 44 and 57 [2]. By the year 2025, it is projected that the global population of postmenopausal women will surpass 1 billion [3]. Due to the drop in estrogen levels during this transition, women may confront symptoms such as vaginal dryness, weight gain, and hair thinning [1]. Furthermore, the onset of menopause is associated with an elevated risk for several health concerns, including lipid metabolic disorders, cardiovascular disease, and osteoporosis [4–6]. Women who have entered the postmenopausal phase often undergo substantial physiological and hormonal shifts that can significantly impact their daily routines. As a result, many opt for hormone therapy to manage these changes. Studies have shown that exogenous estrogen supplementation, such as oral contraceptives, can increase the risk of developing breast and ovarian cancer [7, 8]. Estrogen, progesterone and their receptors are closely associated with the incidence of breast and ovarian cancers, a fact that has been established by extensive epidemiological and experimental research [7, 9].
Breast cancer is the most frequently diagnosed form of cancer among women and also the primary cause of cancer-related mortality [10]. It is projected that approximately one in every 20 women will develop breast cancer at some point in their lifetime [11]. Ovarian cancer ranks as the seventh most prevalent cancer globally, with significant geographical disparities in its incidence. Often detected at advanced stages, it carries a poor prognosis, earning it the distinction of being the deadliest gynecological malignancy [12]. In 2022, the Global Cancer Observatory estimated 20 million new cancer cases and 9.7 million cancer-related deaths worldwide, with breast and ovarian cancers accounting for approximately 13.2% of all diagnoses and nearly 9% of global cancer-related deaths [10]. This data underscores the growing prevalence of breast and ovarian cancers on a global scale. There are differences in the trends of two cancers burden before and after menopause. The ovarian cancer burden in younger women remained relatively unchanged, whereas the burden in postmenopausal and older women is becoming more severe [13]. Studies indicate that between 1990 and 2021, the global disability-adjusted life years (DALYs) of breast cancer in women over 70 reduced [14]. In contrast, the age-standardized mortality rate (ASMR) and age-standardized years of life lost for women aged 15–39 increased [15].
A recent study comprehensively assessed the global burden of gynecological cancer between pre- and post-menopausal from 1990 to 2019 highlighting disparities, but did not address variations of breast cancer [16]. The health, economic, and fertility impacts of breast and ovarian cancers differ significantly between the two age groups, with premenopausal women facing additional burdens such as potential permanent menopause due to some ovarian cancer treatments [16]. There is a gap in research regarding the global burden and future trends of estrogen-related cancers stratified by menopausal status. In this research, we concentrate on the two predominant estrogen-related cancers affecting women – breast cancer and ovarian cancer. Our goal is to assess the patterns and trends in their incidence, DALYs, and mortality rates, and to forecast the development trends up to 2035 for both pre- and post-menopausal women. This analysis aims to inform policies and strategies related to estrogen-related cancers.
Material and methods
Data source and collection
The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2021 dataset covers a wide array of geographical scopes, ranging from the global perspective to continental views across Africa, the Americas, Asia, and Europe, and extends to the national level, encompassing 204 distinct countries and territories [17]. In this analysis, we gathered data on female estrogen-related cancers utilizing the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool). The dataset includes women over the age of 15 across 204 nations, spanning from 1990 to 2021. While estrogen-related cancers in women show a wide range of occurrences, including breast cancer, endometrial cancer, and ovarian cancer, the GBD 2021 dataset only offers burden estimates for two predominant types: breast cancer and ovarian cancer. For example, estrogen can promote the proliferation of the endometrium, thereby contributing to the occurrence of endometrial cancer [18]. However, the GBD database only includes uterine cancer (which encompasses both endometrial cancer and uterine sarcoma), and therefore we did not include it.
Our study population comprised women diagnosed with breast cancer (defined with the International Classification of Diseases version 10 [ICD-10] code C50-C50.9, D05-D05.9, D24-D24.9, D48.6, D49.3), and ovarian cancer (ICD-10 code C56-C56.9, D27-D27.9, D39.1) [17]. Several studies indicate that the median age at natural menopause varies across populations, generally ranging between 45 and 51.5 years [19, 20]. In large-scale epidemiological studies, menopausal status is often defined using age-based criteria due to the lack of individual-level clinical data across diverse populations [21]. This approach is consistent with WHO standards and facilitates comparative burden assessments [22]. In the present study, premenopausal women were defined as those aged 15–49 years, and postmenopausal women as those aged ≥ 50 years. This classification aligns with previous GBD-related analyses on reproductive health and allows for consistent estimation of estrogen-related cancer burden across different demographic groups.
Statistical analysis
In our research, we determined the age-standardized burden of estrogen-related cancers per 100,000 population, encompassing both the age-standardized incidence rate (ASIR) and the ASMR. These rates were computed employing the formula outlined below [23]:
In the formula, αi signifies the age-specific rate for the ith age subgroup, while Wi corresponds to the population count within the same age stratum of the GBD’s world standard population, as derived from the GBD Study 2021 Population data. We determined the estimated annual percentage change (EAPC) in the age-standardized rate (ASR) to assess the average trends over a defined time period [24].
In our analysis, we used local regression models (the “geom_smooth” function in “ggplot2”) to explore the correlation between female estrogen-related cancer burdens and the socio-demographic index (SDI) across 21 regions and 204 countries. We also applied Spearman correlation analysis to determine the correlation coefficients (r) and statistical significance (p) for these relationships in 2021. Additionally, we projected the ASIR and ASMR from 2021 to 2035 using the Bayesian age-period-cohort (BAPC) model [25]. The BAPC model analyzes and predicts disease burden trends by integrating prior knowledge with observed data through Bayesian inference. This approach combines a priori probability distributions of period and cohort effects with sample information to derive posterior distributions, simultaneously estimating age, period, and cohort effects via varying-order random walks [26]. Compared to conventional methods relying solely on sample statistics, BAPC offers greater flexibility in parameter and prior distribution specification, yielding more robust and reliable predictions [27]. Population data was standardized using the WHO World Standard Population database (https://seer.cancer.gov/stdpopulations/world.who.html), while population forecasts (2017–2100) originated from the Global Burden of Disease Study 2019 [28]. Results were expressed as estimates per 100,000 population with 95% uncertainty intervals (UI), 95% certainty intervals (CI) and p < 0.05 was considered statistically significant. All analyses were conducted using R version 4.3.2.
Results
Breast cancer
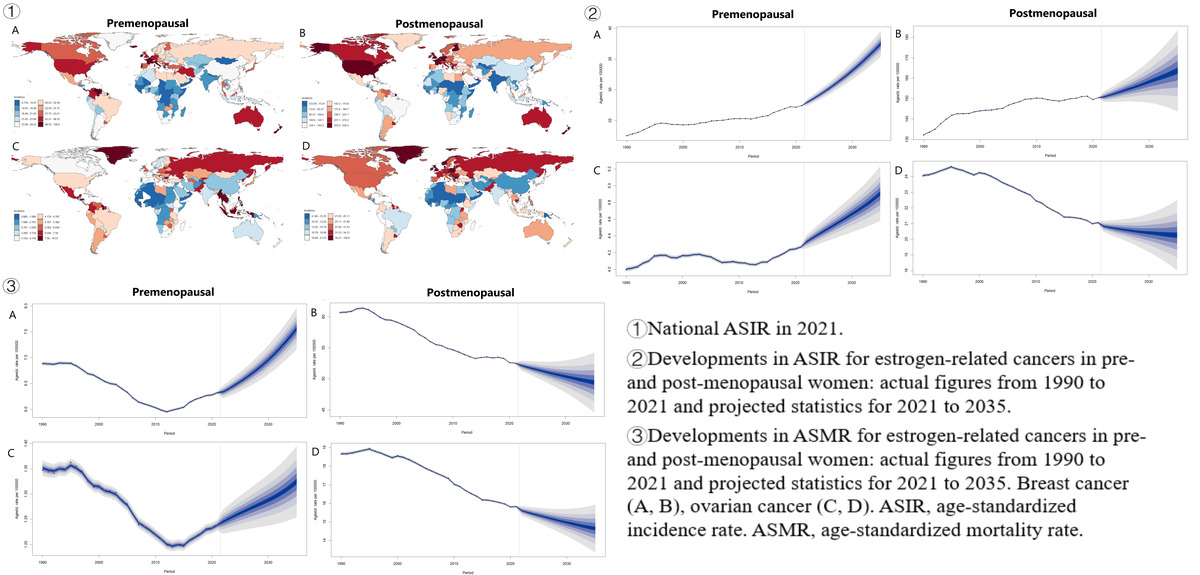
In 2021, the incidence of breast cancer, shows a correlation with SDI, with higher SDI regions experiencing a greater proportion of pre- and post-menopausal cases. The ASIR for both pre- and post-menopausal breast cancer were highest in regions with high SDI. Globally, there were 2,082,736 new female breast cancer cases, with an ASIR of 27.51 (95% UI: 25.46–29.75) for premenopausal and 150.30 (95% UI: 135.72–162.45) for postmenopausal cases (Table I). This led to 129,406 deaths among premenopausal and 531,520 deaths among postmenopausal women (Table I). In 21 GBD regions and 204 countries, premenopausal ASIR peaked in High-income North America (47.20) and Monaco (109.01), postmenopausal in High-income North America (328.03) and United Arab Emirates (530.29; Figures 1 and 2). Premenopausal age-standardized DALY rates and ASMR were highest in Oceania and Nauru, postmenopausal in Southern Sub-Saharan Africa and United Arab Emirates (Figure 1, Supplementary Figures S1, S3 and S4).
Table I
Incidence and deaths of estrogen-related cancers in 2021, and their estimated annual percentage changes from 1990 to 2021. ASIR, age-standardized incidence rate. ASMR, age-standardized mortality rate. EAPC, estimated annual percentage change. SDI, socio-demographic index
Figure 1
Age-standardized incidence and mortality rates in 2021 for estrogen-related cancers, globally and by 21 GBD regions. Age-standardized rates (ASR) of incidence (A, B), and age-standardized rates of deaths (C, D)
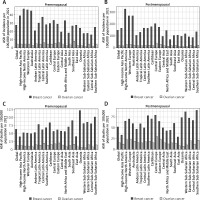
Figure 2
National age-standardized incidence rates in 2021. Breast cancer (A, B), ovarian cancer (C, D)

Between 1990 and 2021, global ASIR for both pre- and post-menopausal breast cancer rose, with the EAPC of 0.50% for premenopausal and 0.36% for postmenopausal cases (Table I). Over three decades, there was a global downward trend in age-standardized DALY rates and ASMR for both groups. For premenopausal women, the age-standardized DALY rate decreased by an EAPC of –0.38%, and ASMR by –0.47% (Table I). Postmenopausal women experienced a more pronounced decline, with age-standardized DALY rates falling by -0.50% and ASMR by –0.57% EAPC (Table I, Supplementary Table SI). Apart from the High SDI region, the incidence of breast cancer has increased in all other SDI regions, for both pre- and post-menopausal women. Across the regional and national levels, the most rapid increases of ASIR for both pre- and post-menopausal women were observed in North Africa and Middle East, Turkey (Supplementary Figure S2). The steepest declines in both ASMR and age-standardized DALY rates for premenopausal women were noted in Western Europe. Conversely, the most significant reductions for postmenopausal women were seen in Australasia and High-income North America (Supplementary Figures S1 and S2).
Ovarian cancer
In 2021, there were 298,876 new ovarian cancer cases globally. The ASIR was 4.27 (95% UI: 3.69–4.78) for premenopausal women and 21.04 (95% UI: 18.69–23.03) for postmenopausal women (Table I). The ASMR was 1.24 (95% UI: 1.08–1.38) for premenopausal and 15.78 (95% UI: 13.97–17.30) for postmenopausal women. Premenopausal ASIR peaked in Southeast Asia (8.38) and Seychelles (16.57), while postmenopausal ASIR was highest in Central Europe (37.01) and United Arab Emirates (100.81; Figures 1 and 2). DALY rates and ASMR were highest in Eastern Europe and Bahamas for premenopausal women, and in Central Europe and United Arab Emirates for postmenopausal women (Figure 1, Supplementary Figures S1, S3 and S4).
From 1990 to 2021, the global ASIR for ovarian cancer increased by 0.07% in premenopausal women but decreased by –0.56% in postmenopausal women (Table I). DALY rates and ASMR declined for both groups, with premenopausal women seeing a –0.37% drop in DALY rates and –0.44% in ASMR, and postmenopausal women experiencing a –0.67% drop in DALY rates and –0.65% in ASMR (Supplementary Table SI, Table I). ASIR and DALY rates increased in low SDI regions but decreased in high and high-middle SDI regions. The sharpest increases in premenopausal ASIR were in Andean Latin America and Ecuador, while the largest decreases in postmenopausal ASIR were in Australasia and Australia (Supplementary Figure S2). The most significant declines in ASMR and DALY rates for premenopausal women were in Australasia and Denmark, and for postmenopausal women, in Australasia and Australia (Supplementary Figures S1 and S2).
Impact of SDI on ASIR, ASMR and DALYs
In 2021, across 204 countries, breast cancer ASIR increased with rising SDI for both pre- and post-menopausal women, as did ovarian cancer ASIR in post-menopausal women. Premenopausal ovarian cancer ASIR rose initially but declined after an SDI of 0.75 (Figure 3). Similar trends were observed for ASMR and DALY rates for breast cancer and premenopausal ovarian cancer, which stabilized or increased before declining at an SDI of 0.75. However, postmenopausal ovarian cancer ASMR continued to rise with increasing SDI (Supplementary Figures S5 and S6). Correlation analyses showed strong positive correlations between ASIR for both cancers and ASMR for ovarian cancer with SDI (r > 0, p < 0.05; Figure 3, Supplementary Figure S5). Conversely, a negative correlation was found between premenopausal breast cancer ASMR and SDI (r < 0, p < 0.05), while no significant association was observed for postmenopausal breast cancer ASMR and SDI.
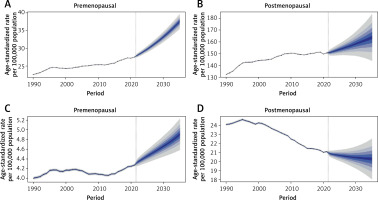
Forecasting ASIR, ASMR and DALYs for estrogen-related cancers from 2021 to 2035
By 2035, the global ASIR for female estrogen-related cancers is likely to rise, except for postmenopausal ovarian cancer, which will decline to 20.26 cases per 100,000 (95% CI: 17.58–22.94) (Figure 4). Premenopausal breast and ovarian cancers are projected to reach incidence rates of 37.49 (95% CI: 35.01–39.96) and 4.91 (95% CI: 4.52–5.29) cases per 100,000, respectively, while postmenopausal breast cancer is forecasted at 163.59 (95% CI: 139.87–187.31) cases per 100,000 (Figure 4). The ASMR for premenopausal estrogen-related cancers is expected to increase from 2021 to 2035, with projected rates of 7.56 (95% CI: 7.08–8.03) for breast cancer and 1.32 (95% CI: 1.24–1.41) for ovarian cancer per 100,000 (Supplementary Figure S7). In contrast, ASMRs for postmenopausal cancers are predicted to decline to 49.42 (95% CI: 43.70–55.15) for breast cancer and 14.64 (95% CI: 13.10–16.17) for ovarian cancer. DALY rates for premenopausal cancers are anticipated to rise, while those for postmenopausal cases are expected to fall (Supplementary Figure S8). To validate the reliability of the BAPC model, we performed an out-of-sample projection exercise. Using only the data from 1990–2014 for premenopausal breast and ovarian cancers, we predicted the age-standardized incidence rates for the year 2021 and then compared these projections with the observed rates (Supplementary Figure S9). The predicted 2021 ASIR of premenopausal breast cancer is 28.48 (95% CI: 27.23–29.74) per 100,000 and the observed 2021 rate is 27.43 (95% CI: 27.36–27.50) per 100,000. The observed value fell well within the 95% prediction interval. The predicted 2021 ASIR of premenopausal ovarian cancer is 4.37 (95% CI: 4.16–4.59) per 100,000 and the observed 2021 rate is 4.27 (95% CI: 4.24–4.30) per 100,000. Again, the observed rate lay entirely within the predicted credible interval. These results indicate that the BAPC model produces accurate and well-calibrated forecasts and support its reliability for the present analysis.
Estrogen-related cancers in pre- and post-menopausal women: age patterns
Supplementary Figure S10 shows the global number, incidence, mortality, and DALY rates for female estrogen-related cancers in 5-year age groups. In 2021, the numbers of incident cases, deaths, and DALYs attributable to female breast cancer rose with age until 55–59 years, then declined. A similar pattern was observed for ovarian cancer: both incident cases and DALYs increased up to 55–59 years and decreased thereafter, whereas deaths continued to climb until 65–69 years before tapering off. When expressed as incidence rates, both cancers increased steadily with age, peaking at 85–89 years for breast cancer and at 90–94 years for ovarian cancer. Mortality rates for both malignancies also rose monotonically with age. The age-specific DALY rate for breast cancer increased progressively, plateauing between 55 and 79 years, whereas the DALY rate for ovarian cancer peaked at 70–74 years and declined afterward.
Discussion
This study offers the first comprehensive analysis of the incidence, DALYs, and mortality of estrogen-related cancers by menopausal status globally. Key findings include: firstly, the ASIR for breast cancer increased globally from 1990 to 2021 in both pre- and post-menopausal women, while postmenopausal ovarian cancer ASIR declined. DALY rates and ASMR for both cancers decreased globally. Second, breast cancer is more prevalent than ovarian cancer, with significant regional variations in burden. Patterns differ between pre- and post-menopausal women. Third, in 2021, ASIR for both cancers and ASMR for ovarian cancer positively correlated with SDI, while premenopausal breast cancer ASMR negatively correlated with SDI. Lastly, from 2021 to 2035, ASIR for pre- and post-menopausal breast cancer is projected to rise, while postmenopausal ovarian cancer ASIR will decline. ASMR and DALYs for premenopausal cancers are expected to increase, while those for postmenopausal cancers are anticipated to decrease.
We found that the ASIR for breast cancer in women both before and after menopause rose markedly on a global scale from 1990 to 2021. In 2021, there were 2,082,737 new female breast cancer cases globally, with 561,438 premenopausal and 1,521,299 postmenopausal cases, trailed by ovarian cancer. This finding aligns with previous studies, highlights the need for heightened awareness and action [10, 14, 15]. Amidst rapid societal progress, numerous studies have reported a correlation between detrimental lifestyle habits and the rising incidence of estrogen-related cancers [29, 30]. For example, obesity and diabetes are two rapidly increasing diseases in the field of endocrinology and metabolism, leading to increased medical and social burdens. Overweight and obesity were correlated with the risk of developing breast and ovarian cancer [30]. The ovaries are the primary source of estrogen in the body, yet in postmenopausal women, adipose tissue contributes a modest amount of this hormone. This additional estrogen may contribute to elevated levels, thereby increasing the susceptibility to breast cancer [7, 9]. Moreover, studies have shown that exogenous estrogen supplementation, such as oral contraceptives, can increase the risk of developing breast and ovarian cancer [7, 8]. High blood glucose was also identified as a potential risk factor attributable to breast cancer [31]. With the advancement of medical technology, the screening and early detection of tumors have become possible, which may partly contribute to the incidence rate of tumors [32]. While therapeutic advances drive mortality declines, rising incidence – fueled by obesogenic environments and reproductive shifts – threatens to outpace gains, especially in rapidly developing economies where lifestyle transitions precede healthcare strengthening. To address this, it is imperative to promote healthy lifestyle behaviors to reduce the risk of estrogen-related cancers. Comprehensive strategies that include public health education, accessible healthcare, and ongoing research are crucial to mitigate the growing cancer burden.
The prevalence and death of estrogen-related cancers exhibit significant variation across 204 countries and territories, underscoring the disparities in prevention, management, and treatment strategies. Our research has identified a robust positive correlation between the ASIR and the SDI in 2021, indicating a higher burden in regions with greater development. Conversely, a notable negative correlation was observed between the ASMR for premenopausal breast cancer in relation to the SDI, suggesting that mortality rates possibly due to socio-economic factors. In high-SDI countries, advancements in diagnosis and treatment have led to a reduction in disability rates and a concurrent increase in cure rates for estrogen-related cancers. Advanced medical infrastructure and enhanced diagnostic capacity in high-income settings may increase case detection and reporting completeness, whereas suboptimal healthcare systems in low-resource settings likely contribute to elevated mortality rates [33, 34]. Evidence indicates that women with lower income and educational attainment exhibit reduced mammography utilization [35], and those with low socioeconomic status demonstrate poorer screening adherence coupled with a twofold increased risk of advanced-stage breast cancer diagnosis compared to their high-socioeconomic-status counterparts [36, 37]. High-income North America faces a significant burden of breast cancer and Central Europe for ovarian cancer. To counter the regional disparities in estrogen-related cancer, it is essential to implement tailored health policies and targeted screening programs that consider local prevalence and risk factors. While enhanced detection contributes to rising ASIR – particularly in high-SDI regions with screening saturation – stage distribution shifts and persistent increases in low-resource settings confirm a genuine epidemiological transition. Future cancer control must address both biological drivers and diagnostic equity.
Worldwide, the burden patterns of estrogen-related cancer differ significantly between pre- and post-menopausal women. Our research reveals that the prevalence of estrogen-related cancer among premenopausal women is typically lower than that among postmenopausal women. There is a significant correlation between the hormonal shifts experienced by postmenopausal women and the increased prevalence of abdominal obesity and glucose intolerance [38], which in turn contributes to a higher likelihood of estrogen-related cancer within this demographic. At the same time, aging affects the occurrence of two estrogen-related cancers through different mechanisms, associated with a higher incidence among postmenopausal women [8, 39]. Utilizing the BAPC model, we project an upward trajectory in the ASMR and DALYs for premenopausal estrogen-related cancers. In contrast, we anticipate a downward trend in the ASMR and DALYs for postmenopausal estrogen-related cancers from 2021 to 2035. The health, economic, and fertility impacts of these cancers differ significantly between the two age groups, with premenopausal women facing additional burdens such as potential permanent menopause due to some ovarian cancer treatments [24]. The financial burden of cancer treatment on adolescents and young adults is profound and enduring, with this demographic being uniquely susceptible to its adverse economic impacts, or financial toxicity [40]. During the postmenopausal stages, women experience a decrease in the levels of estrogen and progesterone produced by the ovaries, signifying the conclusion of their reproductive years and the permanent shutdown of ovarian activity [41]. In conclusion, the shifting epidemiological trends of estrogen-related cancers, coupled with a consistent rise in early-onset cases, are increasingly raising concerns. To alleviate this burden, public health policies can significantly reduce the financial burden on adolescents and young adults with cancer, improving their quality of life and overall well-being.
Menopause is not merely an age milestone but a fundamental biological watershed that dramatically alters the endocrine environment [42]. Our findings robustly confirm that pre- and post-menopausal cancers are, in effect, distinct epidemiological entities. This stark dichotomy underscores that the driver of these trends is likely different: for premenopausal women, factors may include shifting reproductive patterns and early-life exposures, whereas for postmenopausal women, the effectiveness of screening, hormone replacement therapy use, and advancements in treatment may be more influential. To effectively address this challenge, healthcare systems must evolve from reactive treatment models toward more predictive, preventive, and personalized approaches. A comprehensive, integrated program that spans the care continuum – from risk assessment and education to early detection and treatment – is essential. Such a program should begin with enhanced risk stratification tools that incorporate genetic, hormonal, lifestyle, and regional factors, tailored to menopausal status [43, 44]. For example, premenopausal women may benefit from early genetic counseling and regular imaging, whereas postmenopausal women might require more frequent and specialized screening modalities. Implementing digital health tools and artificial intelligence for imaging analysis could improve diagnostic accuracy and allow for scalable, region-specific screening strategies, especially in resource-variable settings. Moreover, healthcare systems should strengthen interdisciplinary collaboration among gynecologists, oncologists, radiologists, and primary care providers to facilitate seamless transitions between prevention, diagnosis, and treatment. Public health initiatives must also focus on increasing awareness of symptoms and risk factors specific to both pre- and post-menopausal populations, particularly in regions with rising incidence rates.
Our research acknowledges certain limitations. Primarily, the absence of comprehensive data on estrogen-related cancers in the GBD database has constrained our analysis to two prevalent types: breast and ovarian cancers, thereby omitting other estrogen-related malignancies. For example, estrogen can promote the proliferation of the endometrium, thereby contributing to the occurrence of endometrial cancer [33]. However, the GBD database only includes uterine cancer (which encompasses both endometrial cancer and uterine sarcoma), and therefore we did not include it. Secondly, the determination of menopausal status in our study relied on age as a surrogate for actual menopause data, a method that could potentially lead to the misclassification of certain cases. We acknowledge that using age 50 as a surrogate for menopausal status is an oversimplification that does not capture biological or regional heterogeneity in menopause onset. Regrettably, this approach was necessary due to the absence of systematic collection of menopause details in cancer registries. However, this method is standard in comparable population-level analyses and allows for comparative assessment across regions and time periods where clinical data is unavailable. Future studies incorporating biomarker data or individual health records could provide more precise estimations. WHO reports 15–49 years as reproductive age while postmenopausal cases as those in women aged 50 and above [22]. And several studies indicate that the median age at menopause varies between 45 and 51.5 years across different populations [19, 20]. We attempted to perform a subgroup analysis; Supplementary Figure S10 presents the global counts, incidence rates, mortality rates, and DALY rates for female estrogen-related cancers stratified by 5-year age groups. Thirdly, the quality of data from the GBD database, along with the methods of data cleaning and statistical analysis employed, may have influenced our disease estimations. The GBD database, while comprehensive, is not immune to uncertainties and variations in data quality across different countries and regions. Lastly, the observed SDI-cancer correlations may reflect unmeasured confounders including regional disparities in healthcare access, screening protocols, and hormone therapy utilization, rather than direct causal effects.
In conclusion, estrogen-related cancers, including breast and ovarian cancer for pre- and post-menopausal, continue to pose a significant global health challenge, with increasing burdens in observed worldwide. The burden of estrogen-related cancer varies considerably across the globe. The upward trend in the burden for pre- and post-menopausal cancers, along with the projected increase in burden from 2021 to 2035, underscores the urgency for targeted public health interventions. Our findings demand menopause-stratified precision public health: High-SDI regions must invest in genetic-risk based prevention (e.g., breast cancer susceptibility gene profiling at premenopausal age) and extend targeted therapy access. Low-SDI regions require urgent scaling of low-cost screening (e.g., handheld ultrasound) and essential medicine supply chains. Implementing this dual-track strategy could avert estrogen-related cancer deaths by 2035.