Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
OSTEOPOROSIS / CLINICAL RESEARCH
YTHDF3-mediated m6A modification facilitates osteosarcoma progression through the FSP1-CoQ10-NAD(P)H axis to suppress ferroptosis
1
Department of Orthopaedics, The Second Affiliated Hospital of Anhui Medical University, China
2
Institute of Orthopaedics, Research Center for Translational Medicine, The Second Affiliated Hospital of Anhui Medical University, China
3
Emergency Department, Anhui No.2 Provincial People's Hospital, China
These authors had equal contribution to this work
Submission date: 2023-11-10
Final revision date: 2024-03-06
Acceptance date: 2024-04-11
Online publication date: 2024-04-19
Corresponding author
Junfeng Zhan
Department of Orthopaedics, The Second Affiliated Hospital of Anhui Medical University, China
Department of Orthopaedics, The Second Affiliated Hospital of Anhui Medical University, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
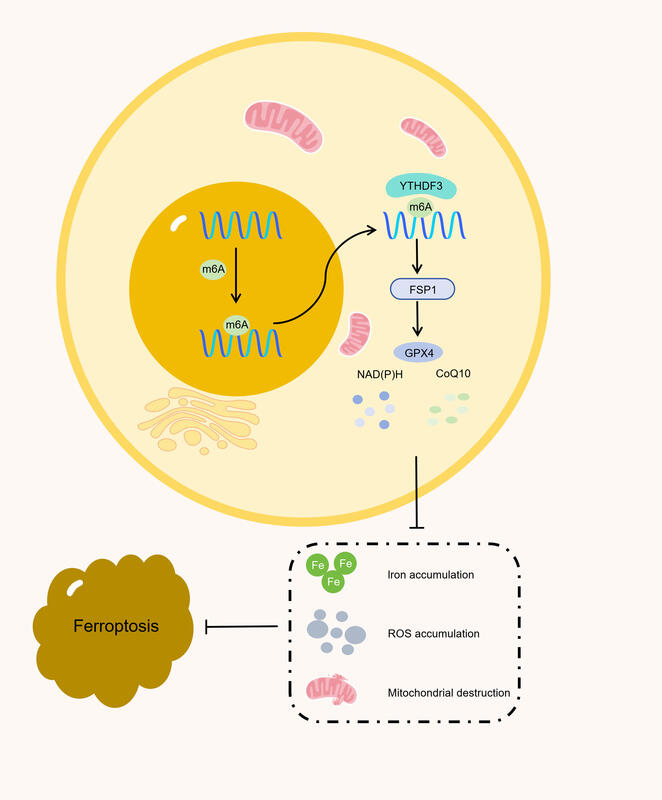
Osteosarcoma (OS) remains a formidable malignancy, characterized by its relentless nature and limited therapeutic interventions. YTHDF3, a key reader protein recognizing m6A-modified mRNAs, has attracted considerable attention due to its prominent role in cancer biology. This study investigated the relationship between YTHDF3, a vital RNA modification reader protein, m6A RNA modification, and the FSP1-CoQ10-NADPH metabolic pathway in the pathogenesis of OS.
Material and methods:
Firstly, we procured tissue specimens and corresponding non-cancerous tissues from 10 OS patients and cultured OS cell lines. Then, we established a ferroptosis model in OS cells through treatment with RSL3 to reveal the relation between YTHDF3 and ferroptosis.
Results:
Clinical evaluation of OS samples revealed a notable upsurge in the expression of YTHDF3 and the concurrent overexpression of ferroptosis-related proteins. In vitro experiments suggested that YTHDF3 potentially facilitated FSP1 mRNA translation through an m6A-dependent mechanism, subsequently inhibiting ferroptosis via the FSP1-CoQ10-NADPH pathway, thereby promoting OS progression. These compelling findings underscore the promise of targeting the YTHDF3-FSP1 axis as an innovative and potentially transformative therapeutic strategy for the treatment of OS.
Conclusions:
This study not only enhances our understanding of ferroptosis regulation but also sheds light on the significance of YTHDF3 and FSP1 as potential targets for therapeutic intervention in OS, offering new prospects for cancer treatment strategies.
Osteosarcoma (OS) remains a formidable malignancy, characterized by its relentless nature and limited therapeutic interventions. YTHDF3, a key reader protein recognizing m6A-modified mRNAs, has attracted considerable attention due to its prominent role in cancer biology. This study investigated the relationship between YTHDF3, a vital RNA modification reader protein, m6A RNA modification, and the FSP1-CoQ10-NADPH metabolic pathway in the pathogenesis of OS.
Material and methods:
Firstly, we procured tissue specimens and corresponding non-cancerous tissues from 10 OS patients and cultured OS cell lines. Then, we established a ferroptosis model in OS cells through treatment with RSL3 to reveal the relation between YTHDF3 and ferroptosis.
Results:
Clinical evaluation of OS samples revealed a notable upsurge in the expression of YTHDF3 and the concurrent overexpression of ferroptosis-related proteins. In vitro experiments suggested that YTHDF3 potentially facilitated FSP1 mRNA translation through an m6A-dependent mechanism, subsequently inhibiting ferroptosis via the FSP1-CoQ10-NADPH pathway, thereby promoting OS progression. These compelling findings underscore the promise of targeting the YTHDF3-FSP1 axis as an innovative and potentially transformative therapeutic strategy for the treatment of OS.
Conclusions:
This study not only enhances our understanding of ferroptosis regulation but also sheds light on the significance of YTHDF3 and FSP1 as potential targets for therapeutic intervention in OS, offering new prospects for cancer treatment strategies.
REFERENCES (57)
1.
Nørregaard KS, Jürgensen HJ, Gårdsvoll H, Engelholm LH, Behrendt N, Søe K. Osteosarcoma and metastasis associated bone degradation - a tale of osteoclast and malignant cell cooperativity. Int J Mol Sci 2021; 22: 6865.
2.
Zhao Z, Lin X, Tong Y, Li W. Silencing lncRNA ZFAS1 or elevated microRNA-135a represses proliferation, migration, invasion and resistance to apoptosis of osteosarcoma cells. Cancer Cell Int 2019; 19: 326.
3.
Kudarha R, Dhas N, Mutalik S. Distinct features of iron based metal organic frameworks (MOFs) for ferroptosis mediated cancer therapy: a comprehensive review. Coord Chem Rev 2023; 494: 215330.
4.
Lin KJ, Chen SD, Lin KL, et al. Iron brain menace: the involvement of ferroptosis in Parkinson disease. Cells 2022; 11: 3829.
5.
Lee J, Roh JL. Unleashing ferroptosis in human cancers: targeting ferroptosis suppressor protein 1 for overcoming therapy resistance. Antioxidants 2023; 12: 1218.
6.
Guo J, Chen L, Ma M. Ginsenoside Rg1 suppresses ferroptosis of renal tubular epithelial cells in sepsis-induced acute kidney injury via the FSP1-CoQ10-NAD (P) H pathway. Curr Med Chem 2023; 31: 2119-32.
7.
Li W, Liang L, Liu S, Yi H, Zhou Y. FSP1: a key regulator of ferroptosis. Trends Mol Med 2023; 29: 753-64.
8.
Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci 2021; 276: 119399.
9.
Sun Y, Jin D, Zhang Z, et al. N6-methyladenosine (m6A) methylation in kidney diseases: mechanisms and therapeutic potential. Biochim Biophys Acta Gene Regul Mech 2023; 1886: 194967.
10.
Chen D, Cheung H, Lau HCH, Yu J, Wong CC. N6-methyladenosine RNA-binding protein YTHDF1 in gastrointestinal cancers: function, molecular mechanism and clinical implication. Cancers 2022; 14: 3489.
11.
Chang LL, Xu QG, Liu XL, et al. Emerging role of m6A methylation modification in ovarian cancer. Cancer Cell Int 2021; 21: 663.
12.
Yang Z, Cai Z, Yang C, Luo Z, Bao X. ALKBH5 regulates Stat3 activity to affect the proliferation and tumorigenicity of osteosarcoma via an m6A-YTHDF2-dependent manner. eBioMedicine 2022; 80: 104019.
13.
Li HB, Huang G, Tu J, et al. METTL14-mediated epitranscriptome modification of MN1 mRNA promote tumorigenicity and all-trans-retinoic acid resistance in osteosarcoma. EBioMedicine 2022; 82: 104142.
14.
Xu Y, Zhang W, Shen F, et al. YTH domain proteins: a family of m6A readers in cancer progression. Front Oncol 2021; 11: 629560.
15.
Yu Y, Meng LL, Chen XY, et al. m6A reader YTHDF3 is associated with clinical prognosis, related RNA signatures and immunosuppression in gastric cancer. Cell Signal 2023; 108: 110699.
16.
Ni W, Yao S, Zhou Y, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer 2019; 18: 143.
17.
Pillai-Kastoori L, Schutz-Geschwender AR, Harford JA. A systematic approach to quantitative Western blot analysis. Anal Biochem 2020; 593: 113608.
18.
Li S, He Y, Chen K, et al. RSL3 drives ferroptosis through NF-B pathway activation and GPX4 depletion in glioblastoma. Oxid Med Cell Longev 2021; 2021: 2915019.
19.
Wang DP, Tang XZ, Liang QK, et al. microRNA-599 promotes apoptosis and represses proliferation and epithelial-mesenchymal transition of papillary thyroid carcinoma cells via downregulation of Hey2-depentent Notch signaling pathway. J Cell Physiol 2020; 235: 2492-505.
20.
Zeng F, Ye l, Zhou Q, et al. Inhibiting SCD expression by IGF1R during lorlatinib therapy sensitizes melanoma to ferroptosis. Redox Biol 2023; 61: 102653.
21.
Chen Y, Chen Y, Zhang H, Wang T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult Sci 2020; 99: 3158-67.
22.
Ma Y, Li Y, Ling S, et al. Loss of heterozygosity for KrasG12D promotes REDD1-dependent, non-canonical glutamine metabolism in pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun 2020; 526: 880-8.
23.
Chai Y, Cao ZM, Yu R, et al. Dexmedetomidine attenuates LPS-induced monocyte-endothelial adherence via inhibiting Cx43/PKC-/NOX2/ROS signaling pathway in monocytes. Oxid Med Cell Longev 2020; 2020:.
24.
Nikaido Y, Midorikawa Y, Furukawa T, et al. The role of neutrophil gelatinase-associated lipocalin and iron homeostasis in object recognition impairment in aged sepsis-survivor rats. Sci Rep 2022; 12: 249.
25.
Deng X, Yang P, Gao T, Liu M, Li X. Allicin attenuates myocardial apoptosis, inflammation and mitochondrial injury during hypoxia-reoxygenation: an in vitro study. BMC Cardiovasc Disord 2021; 21: 200.
26.
Bornaque F, Delannoy CP, Courty E, et al. Glucose regulates m6A methylation of RNA in pancreatic Islets. Cells 2022; 11: 291.
27.
Xia P, Zhang H, Xu K, et al. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma. Cell Death Dis 2021; 12: 691.
28.
Park KR, Yun HM, Hong JT. G721-0282 inhibits cell growth and induces apoptosis in human osteosarcoma through down-regulation of the STAT3 pathway. Int J Biol Sci 2020; 16: 330.
29.
Chlipala E, Bendzinski CM, Chu K, et al. Optical density-based image analysis method for the evaluation of hematoxylin and eosin staining precision. J Histotechnol 2020; 43: 29-37.
30.
Handbook of Practical Immunohistochemistry: Frequently Asked Questions. Lin F, Prichard JW, Liu H, Wilkerson ML (eds.). Springer Nature 2022.
31.
Sekhar KR, Hanna DN, Cyr S, et al. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroid cancer. Sci Rep 2022; 12: 19396.
32.
Song T, Yang Y, Jiang S, Peng J. Novel insights into adipogenesis from the perspective of transcriptional and RNA N6-methyladenosine-mediated post-transcriptional regulation. Adv Sci 2020; 7: 2001563.
33.
Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet 2014; 15: 293-306.
34.
Meyer KD. m6A-mediated translation regulation. Biochim Biophys Acta Gene Regul Mechan 2019; 1862: 301-9.
35.
Panneerdoss S, Eedunuri VK, Yadav P, et al. Cross-talk among writers, readers, and erasers of m6A regulates cancer growth and progression. Sci Adv 2018; 4: eaar8263.
36.
He C, He C. m6A RNA methylation: from mechanisms to therapeutic potential. EMBO J 2021; 40: e105977.
37.
Zaccara S, Jaffrey SR. A unified model for the function of YTHDF proteins in regulating m6A-modified mRNA. Cell 2020; 181: 1582-95.
38.
Liu T, Wei Q, Jin J, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res 2020; 48: 3816-31.
39.
Chang G, Shi L, Ye Y, et al. YTHDF3 induces the translation of m6A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell 2020; 38: 857-71.
40.
Ran Y, Yan Z, Jiang B, Liang P. N6-methyladenosine functions and its role in skin cancer. Exp Dermatol 2023; 32: 4-12.
41.
Chen X, Kang R, Kroemer G, Tang D. Organelle-specific regulation of ferroptosis. Cell Death Differ 2021; 28: 2843-56.
42.
Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med 2021; 218: e20210518.
43.
Zeng W, Long X, Liu PS, Xie X. The interplay of oncogenic signaling, oxidative stress and ferroptosis in cancer. Int J Cancer 2023; 153: 918-31.
44.
Sun K, Zhi Y, Ren W, et al. The mitochondrial regulation in ferroptosis signaling pathway and its potential strategies for cancer. Biomed Pharmacother 2023; 169: 115892.
45.
Battaglia AM, Chirillo R, Aversa I, et al. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells 2020; 9: 1505.
46.
Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019; 575: 688-92.
47.
Brown CW, Amante JJ, Chhoy P, et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell 2019; 51: 575-86.e4.
48.
Zhao Y, Liu Y, Xu Y, et al. The role of ferroptosis in blood–brain barrier injury. Cell Mol Neurobiol 2023; 43: 223-36.
49.
Luo M, Yan J, Hu X, et al. Targeting lipid metabolism for ferroptotic cancer therapy. Apoptosis 2023; 28: 81-107.
50.
Su LJ, Zhang JH, Gomez H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev 2019; 2019: 5080843.
51.
Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019; 575: 693-8.
52.
Yang J, Jia Z, Zhang J, et al. Metabolic intervention nanoparticles for triple-negative breast cancer therapy via overcoming FSP1-mediated ferroptosis resistance. Adv Healthc Mater 2022; 11: 2102799.
53.
Bornes L, van Scheppingen RH, Beerling E, et al. Fsp1-mediated lineage tracing fails to detect the majority of disseminating cells undergoing EMT. Cell Rep 2019; 29: 2565-9.e3.
54.
Ni Y, Zhou X, Yang J, et al. The role of tumor-stroma interactions in drug resistance within tumor microenvironment. Front Cell Develop Biol 2021; 9: 637675.
55.
Zhang Z, Wei W, Wang H, Dong J. N6-methyladenosine-sculpted regulatory landscape of noncoding RNA. Front Oncol 2021; 11: 743990.
56.
Feng H, Yuan X, Wu S, et al. Effects of writers, erasers and readers within miRNA-related m6A modification in cancers. Cell Prolif 2023; 56: e13340.
57.
Bu C, Hu S, Yu J, et al. Fear stress promotes glioma progression through inhibition of ferroptosis by enhancing FSP1 stability. Clin Transl Oncol 2023; 25: 1378-88.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.