Introduction
In the last decades, life expectancy has risen steadily in most countries, leading to a growing population [1]. Aging involves a gradual decline in vitality, physical strength, cognitive ability, and overall physiological function [2]. However, disparities often exist between chronological age and biological age (functional capacity). Although physiological functions generally decline with age, the variation in functional status highlights the need for individualized assessment in clinical practice and aging studies [3].
Nearly half of individuals diagnosed with diabetes mellitus (DM) belong to the older population [4]. DM imposes a substantial burden on healthcare systems globally, contributing to morbidity and mortality through its numerous complications and associated comorbidities [5, 6]. Although the literature provides limited data on hospitalization outcomes of very old patients with DM, studies have shown that patients often experience a decline in functional capacity upon discharge compared to admission [7]. Evaluating outcomes and identifying mortality predictors in this population are crucial for optimizing medical care and enhancing healthcare services. Notably, predictors of long-term mortality in older hospitalized adults after discharge – particularly clinical factors such as comorbidities, functional status, laboratory values (e.g., renal function, nutritional markers), and systemic inflammation – remain a topic of considerable interest [8, 9]. However, studies with long-term follow-up in the late elderly population admitted to medical departments, particularly in Greek and southern Mediterranean populations, remain limited.
This prospective study aimed to assess the 3-year post-discharge mortality rate and predictive factors in older patients, identifying possible differences between patients with and without DM.
Material and methods
This prospective cohort study was conducted in the Internal Medicine Department of the University Hospital of Heraklion, Crete, Greece, with patient recruitment taking place between May 2019 and June 2022. Patients aged ≥ 65 years, as per the World Health Organization’s (WHO) definition of older adults, were included in this study [10]. Additionally, according to the WHO, older patients were classified into two groups: “early elderly”, aged 65 to 74 years, and “late/very elderly”, aged over 75 years.
On admission, the following data were recorded: demographic and somatometric characteristics; past medical history (type 2 DM, heart failure (HF), coronary heart disease (CHD), cerebrovascular disease, peripheral artery disease (PAD), chronic kidney disease (CKD), hypertension, dyslipidemia, dementia), chronic medication use (antidiabetic, antihypertensive, hypolipidemic, diuretics, antiplatelet drugs); cause of hospitalization; laboratory parameters on admission (full blood count, serum creatinine, glycated hemoglobin (HbA1c), albumin levels, urine albumin-to-creatinine ratio (UACR)); duration of hospitalization in the year before the current admission; and the occurrence of any previous hospitalizations.
Laboratory analyses were conducted at the Central Laboratory of the University Hospital of Heraklion. HbA1c was measured using high-performance liquid chromatography (HPLC) with the Bio-Rad D-10 analyzer. Serum parameters including albumin, creatinine, C-reactive protein (CRP), and lipid profile components (total cholesterol and low-density lipoprotein (LDL) cholesterol) were determined using standard enzymatic colorimetric methods. Specifically, CRP was measured via immunoturbidimetric assay, LDL cholesterol and total cholesterol were quantified using the CHO-POD enzymatic method, and serum albumin was measured using the bromocresol green dye-binding method. UACR was calculated from spot urine samples, based on urine albumin measured by the pyrogallol red dye-binding method and creatinine quantified by the kinetic Jaffe reaction.
In addition, cognitive status and dependency on activities of daily living (ADL) were assessed using the Katz Index and the Barthel Index (BI), respectively. Patients with a BI score ≤ 61 were identified as having moderate to total disability [11]. Similarly, those with a Katz Index score ≤ 3 were identified as having significant disability [12]. Frailty was assessed using the 5-item Fried Frailty Score (FFS) and Clinical Frailty Scale (CFS). A cutoff score of ≥ 6 for CFS was used to identify those with greater than moderate frailty [13]. Additionally, a cut-off of ≥ 3 for FFS defined frailty [14]. In order to assess morbidity, the Charlson Comorbidity Index (CCI) was estimated; patients with a CCI index ≥ 5 were classified as having severe morbidity [15]. Severity upon admission was also assessed using the qSOFA score. Additionally, outcomes regarding length of hospital stay (LOS), Intensive Care Unit (ICU) transfer, and mortality rates were assessed and evaluated. Discharged patients were followed for 3 years, with data on readmissions and mortality collected through telephone interviews or, when unavailable, from medical records and hospital administrative systems.
The study received ethical approval by the Ethics Committee of the University Hospital of Heraklion. Informed consent was obtained from all patients participating in the study. In cases where a patient was unable to provide consent, consent was obtained from their legal representative.
Statistical analysis
The study population was categorized into two groups: the DM group and non-DM group. Continuous data were analyzed using Student’s t test for normally distributed variables and the Mann-Whitney test for non-normally distributed data. Categorical data were analyzed using the χ2 test when all expected cell counts were ≥ 5, and Fisher’s exact test was applied when expected counts were < 5. Data are presented as number (%) for categorical variables and mean (± standard deviation (SD)) or median (interquartile range (IQR)) for continuous variables. All tests were two-tailed, and a p-value ≤ 0.05 was considered significant.
Logistic regression analysis models were developed in both groups to assess 3-year post-discharge mortality predictors. Age, sex, past medical history, previous hospitalizations within the past year, chronic medication use, laboratory examinations, frailty, and dependency on activities of daily living scores were the examined parameters. Multivariate logistic regression analyses were employed, including the factors having p < 0.05 in the univariate analysis. All the parameters mentioned above were calculated with SPSS version 26.0 (IBM Corp., Armonk, N.Y., USA).
Results
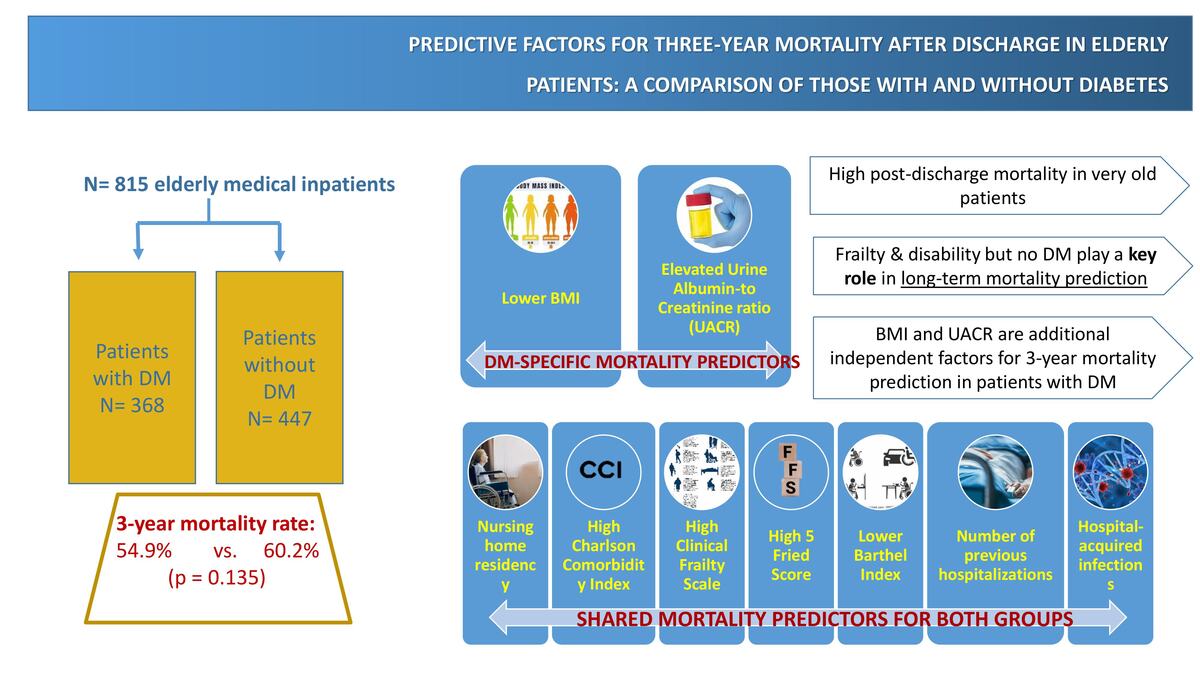
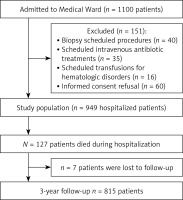
During the study period, 1134 individuals were admitted to the medical ward; of those, n = 34 were < 65 years old and were excluded. Out of 1100 individuals aged ≥ 65 years, 151 were excluded due to scheduled admission or for not providing informed consent, 127 died during hospitalization, and follow-up records were unavailable for seven individuals. Therefore, the working sample for assessing 3-year post-discharge mortality consisted of 815 discharged patients, as outlined in the study’s flowchart (Figure 1). Among these patients, n = 168 (20.6%) were early elderly, and n = 647 (79.4%) were late elderly. In the whole sample, the median age was 83.0 years (IQR 77.0, 88.0), and 375 (46%) were males.
Three hundred sixty-eight (45.2%) individuals had DM with a mean HbA1c level of 6.5 ±1.6 (Table I). The median duration of DM was 13.0 (11.0, 18.0) years. The DM group had a marginally lower median age compared to the non-DM group (82 vs. 85 years, p < 0.01) and a slightly elevated body mass index (BMI) (26.0 vs. 25.0, p < 0.01). The occurrence of any hospitalizations during the year preceding the current admission and the total number of days spent in the hospital during that period were similar between the two groups. The DM group exhibited a numerically greater, but not statistically significant, disability rate based on the BI (mean BI score 64 vs. 54, respectively p = 0.09) and Katz Index (39.1 vs. 42.3, p = 0.39). Regarding frailty, no statistically significant differences were found between those with DM and those without DM as assessed by FFS (33.2% vs. 38.5%, p = 0.12) and CFS (39.7% vs. 45.2%, p = 0.12) (Table I).
Table I
Baseline characteristics, causes of admission, and outcomes
[i] ARBs – angiotensin II receptor blockers, DM – diabetes mellitus, BMI – body mass index, CAD – coronary artery disease, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft, AF – atrial fibrillation, CKD – chronic kidney disease, CFS – clinical frailty scale, ACEi – angiotensin-converting enzyme inhibitors, CCBs – calcium channel blockers, PPIs – proton pump inhibitors, RTI – respiratory tract infection, UTI – urinary tract infection, SSTI – skin and soft tissue infection LOS – length of hospital stay, ICU – intensive care unit, UACR – urine albumin to creatinine ratio, ADPi – adenosine diphospate inhibitors, COX inhibitors-cyclooxygenase inhibitors, SGLT2i – sodium glucose co-transporter 2 inhibitors, GLP1 – glucagone like peptide 1, CRP – C-reactive protein, HbA1c – glycated hemoglobin, AKI – acute kidney injury, LDL – low density lipoprotein, GFR – glomerular filtration rate.
A significantly greater percentage of individuals with DM exhibited increased morbidity, based on CCI, compared to the non-DM group (p < 0.01, Table I). Additionally, patients with DM exhibited a significantly higher prevalence of polypharmacy (p < 0.01), and higher percentages of them received angiotensin-converting enzyme (ACE) inhibitors and statins (p < 0.01) compared to patients without DM.
Regarding causes of admission, anemia and HF decompensation occurred significantly more frequently in those with DM compared to the rest. Higher qSOFA scores on admission were observed in those with DM (p < 0.01).
During admission, the DM group experienced higher rates of hospital-acquired infections (HAIs) (p < 0.01). The use of vasoactive drugs was observed in higher rates in those with DM compared to the rest (6.8% vs. 2.5%, p < 0.01). However, no statistically significant difference was observed in ICU admission. The mean length of hospital stay (approximately 7 days) was similar in both groups. The overall incidence of 3-year post-discharge mortality among the 815 elderly patients was 57.8%. Mortality rates were comparable between the DM and non-DM groups, at 54.9% and 60.2%, respectively (Table I).
In patients who were discharged, no statistically significant differences regarding 3-year mortality rates were observed between DM and non-DM groups (χ2 = 2.31, p = 0.13). Linear regression analyses were performed to identify predictors of 3-year mortality and to assess any differences between patients with and without DM. The results from univariate and multivariate analyses for the DM group are presented in Table II and for the non-DM group in Table III. Nursing home residency, elevated CCI, higher CFS, higher FFS, lower BI, longer duration in the hospital before admission, and HAIs were identified as statistically significant factors of 3-year post-discharge mortality in both groups. In patients with DM, lower BMI (p = 0.03) and increased UACR (p = 0.04) during admission were observed as additional significant 3-year post-discharge mortality predictors.
Table II
Univariate and multivariate analyses for predictive factors of three-year post-discharge mortality in subjects with diabetes (n = 368)
Table III
Univariate and multivariate analyses for predictive factors of three-year post-discharge mortality in subjects without diabetes (n = 447)
[i] BMI – body mass index, RTI – respiratory tract infection, UTI – urinary tract infection, LOS – length of hospital stay, AKI – acute kidney injury, UACR – urine albumin to creatinine ratio, CCI – Charlson Comorbidity Index, FFS – Fried Frailty Score, CFS – Clinical Frailty Scale, ACEi – angiotensin-converting enzyme inhibitors.
Discussion
There is a gap in the literature regarding the late elderly population admitted to medical departments, particularly in Greek and southern Mediterranean populations [16, 17]. To the best of our knowledge, our study is one of the few examining post-discharge mortality in late elderly subjects and has the longest period of observation. Our results indicate that almost 2 out of 3 late elderly patients discharged from a medical department die in the next 3 years. This high mortality rate should be taken into consideration when planning the post-discharge plan and regimen of this population, which includes older, frail individuals with a greater burden of comorbidities. Despite the higher rate of comorbidities, no statistically significant difference in 3-year mortality was observed between patients with and without DM. This is a key finding of this study, indicating that DM in this age group – very old patients – is not independently associated with long-term post-discharge mortality. The post-discharge mortality predictors that were identified in both groups included higher CCI, longer duration of past hospitalization, higher CFS, lower BI, higher FFS, HAIs, and nursing home residency. In the DM group, lower BMI and higher UACR levels were additionally identified as 3-year post-discharge mortality predictors.
Our results align with other studies on elderly hospitalized patients with DM, indicating that DM did not significantly impact mortality [18–21]. In the RepoSI trial, which analyzed data from internal medicine and geriatric wards, diabetes was not a predictor of 1-year mortality [18]. This study contributes to the existing literature by providing long-term mortality data and a direct comparison between groups with DM and non-DM groups. Our cohort findings in a population with high frailty, advanced age, and diabetes duration over 10 years revealed high overall mortality, with DM status not being a critical factor, possibly reflecting advances in DM management and chronic disease care.
Frailty, as measured by the CFS and FFS, emerged as highly significant predictors for long-term mortality in both groups in this study. Previous research has demonstrated that CFS is a strong predictor of mortality in hospitalized patients [22–24]. Similarly, BI was also found to be a predictor of mortality in older patients [25]. Frailty reflects overall vulnerability, and disability reflects the loss of functional capacity. Both factors, as indicated in our study, contribute to mortality risk in late elderly hospitalized patients regardless of the presence of DM. Therefore, these scores have the potential to provide useful information for the planning of treatment and long-term care of older patients. Moreover, this study confirms the evidence of CCI being a strong independent factor of long-term mortality in very elderly patients [22, 26].
Older patients are particularly vulnerable to HAIs due to age-related immune decline, which heightens their susceptibility to infections [27, 28]. This study identified HAIs as factors independently associated with 3-year mortality, irrespective of DM. While DM can increase infection risk and severity, frailty appears to play a more critical role in very old patients [29]. Frailty in this population often leads to prolonged hospital stays, increased morbidity, and a higher risk of in-hospital mortality, underscoring that age-related vulnerability may outweigh the impact of DM on infection outcomes [30, 31].
There is a gap in the literature regarding the impact of prior hospitalizations on long-term mortality in very elderly patients. This study found that the duration of hospitalization the past year before the current admission was a significant predictor of 3-year mortality. More than half of hospitalized older patients experience new functional impairments upon discharge [32]. Each hospitalization is often accompanied by the onset of geriatric syndromes, further complicating patient outcomes and diminishing quality of life [33].
Furthermore, residing in a nursing home was another independent mortality predictor for both groups. According to the literature, heightened risks of mortality, recurrent hospitalizations, and post-discharge complications are observed at higher rates in older individuals living in nursing homes compared to those dwelling in the community [34]. In our analysis, more than half of the residents in nursing homes were classified as frail and disabled, according to the CFS and BI, respectively. Nursing homes typically accommodate patients who are in the later stages of life, often with significant comorbidities and high levels of dependency [34]. Consequently, nursing home residency should be considered an indirect indicator of frailty.
A lower BMI has been identified as an independent factor for increased mortality in older individuals with DM [35, 36]. Notably, it appears that being overweight or mildly obese may confer protective benefits for older adults [37, 38], a phenomenon known as the “obesity paradox”, wherein overweight older individuals may experience better outcomes than their normal-weight or underweight peers [39]. BMI does not accurately capture key aspects such as adiposity distribution, muscle mass, or recent weight changes, factors that are particularly relevant in older adults. As such, BMI should be interpreted alongside more comprehensive nutritional and functional indicators when assessing health risks in the elderly. Sarcopenia is partially associated with the frailty syndrome and is a recognized predictor of adverse outcomes, including increased mortality [40–42]. Protein-energy malnutrition, which disproportionately affects hospitalized older adults, is a major modifiable factor driving muscle loss and functional decline [43–45].
Albuminuria is prevalent among the elderly due to the pathophysiological changes associated with aging [46, 47]. UACR was independently associated with long-term mortality in adults with type 2 DM aged > 65 years old [48]. Our study adds to previous evidence by showing that UACR on admission, despite its possible large variability due to acute illness, is a predictor of 3-year mortality after discharge in very old patients with DM.
In our study population of very old adults with DM, the use of newer antidiabetic agents such as SGLT2 inhibitors and GLP-1 receptor agonists was limited. Although cardioprotective and renoprotective trials of these drug classes have been established, there are limited data in very elderly populations; thus, these drugs might not have been incorporated into routine practice in geriatric populations during the recruitment period of our study. Additionally, concerns regarding tolerability in late elderly patients, including risk of hypotension, dehydration, or genitourinary infections, may have contributed to the limited use of SGLT2 inhibitors in this very old population [49]. Similarly, frailty and risk of unintended weight loss, sarcopenia, or significant gastrointestinal side effects [50] may have restrained GLP-1 agonist use in our sample.
Despite its prospective design and sample size of very old adults, this study has certain limitations. The single-center design of this study may limit the generalizability of its findings to broader populations; however, our hospital admits patients from both urban and rural regions. Moreover, additional scores assessing frailty and nutritional status have been used in the literature, and their incorporation could have further enriched our study results. Acknowledging these limitations is crucial for future research endeavors aimed at better understanding the impact of DM and its comorbidities on health outcomes of very old people.
In conclusion, this study highlights that individuals of very old age have a high 3-year post-discharge mortality rate, whereas DM per se is not a significant contributor to long-term mortality. Frailty and comorbidity, prior hospitalizations during the past year, and nursing home residency were observed as independent predictors of long-term mortality in our sample. Among subjects with DM, lower BMI and elevated UACR on admission were found to be additional 3-year post-discharge mortality factors. These findings highlight the need for regular assessment of frailty and disability in very old hospitalized patients, whereas, in those with DM, the assessment of BMI and UACR may aid in better long-term mortality risk estimation.