Introduction
Breast cancer is the most common malignant tumor and the second leading cause of cancer-related mortality in women worldwide [1, 2]. Invasive ductal carcinoma (IDC), sometimes called infiltrating ductal carcinoma, is the most common type of breast cancer. About 80% of all breast cancers are IDCs [1–3]. However, some other pathological subtypes can occur in patients with IDC. Its histological type reflects the source and malignancy of the tumor and is closely related to the selection of clinical treatment. In 1980, Fisher et al. reported that invasive micropapillary carcinoma (IMPC) was associated with the high invasion and metastasis of the tumor and its micropapillary components were associated with local recurrence [4]. To date, the treatment of IMPC is the same as the standard treatment of nonspecific invasive breast cancer (NSIBC). It remains controversial whether the survival of patients with IMPC is worse or not. IMPC is a rare pathological subtype accounting for 2% to 8% of invasive breast carcinomas [5, 6]. Since Fisher first demonstrated a sample with mulberry morphological changes of invasive papillary carcinoma in 1980 [4], there have been many different reports of IMPC pathological diagnostic standards. The large difference in the reported incidence of IMPC is mainly because, in most cases, IMPC is a component of IDC, and does not represent all components of the cancer. The formal concept of IMPC was initially put forth by Siriaunkgul and Tavassoli in 1993 [7]. Because of its unique morphological characteristics and a higher propensity for invasiveness, IMPC was listed as an independent subtype in the 2003 World Health Organization classification of breast cancer [8]. The typical pathological feature of IMPC is that the tumor cells are arranged in small clusters in the vascular-like interstitial space, and epithelial membrane antigen staining shows cell polarity reversal. Ductal carcinoma in situ (DCIS) is a noninvasive form of breast cancer consisting of malignant cells that do not invade the basement membrane of the breast ducts. The reported percentage of breast cancer patients with DCIS coexisting with IDC varied significantly from 21.3% to 76.9% [9, 10].
Herein, we performed a retrospective analysis on 104 patients with IMPC who met the inclusion criteria among 4532 patients with invasive breast cancer admitted to our hospital in the past 5 years. We selected a cohort of 230 patients with NSIBC as a contemporaneous control using the matching method. We compared the clinicopathological features, treatment, and outcomes of the two cohorts to explore the factors affecting the prognosis of IMPC. To the best of our knowledge, this is the first study to analyze the clinicopathological features of IMPC and to investigate the factors affecting its prognosis. The findings of this study provide a valuable reference for precise clinical treatment and diagnostic evaluation.
Material and methods
Patients
The clinical data of 4532 patients, with complete records, were collected from the General Hospital of Ningxia Medical University between January 2015 and December 2019. Out of these patients, 227 were diagnosed through surgical pathology and 104 of them met the inclusion criteria. Additionally, 230 NSIBC patients were included using a matching method.
Inclusion criteria
The inclusion criteria for this study comprised the following: patients had to be female, with IMPC confirmed via surgical pathology; imaging examinations such as preoperative ultrasound, computed tomography (CT), and electroconvulsive therapy (ECT) had to rule out distant metastasis such as bone, liver, lung or brain; patients received standardized postoperative treatment such as adjuvant chemotherapy, radiotherapy or endocrine therapy; and their medical records and follow-up data had to be complete. Given that the sample groups had different distributions, we matched participants according to their pathological conditions and physical examination using propensity scores.
Exclusion criteria
The exclusion criteria consisted of male gender, presence of other malignant tumors such as synchronous and heterochronous bilateral breast cancer, neoadjuvant treatment patients, or cases that did not match the NSIBC cases. For criteria matching: NSIBC patients who received surgical treatment at the same institution during the same time were independently matched. Additionally, other critical variables such as age were controlled with a difference of 3 years, while tumor size and lymph node stage were precisely matched. To ensure consistency, the topical therapy methods were completely matched based on surgery and radiology types.
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University.
Diagnostic criteria
The diagnostic criteria of IMPC were determined as per the Pathological and Genetic Classification of Breast Cancer and Female Reproductive System Tumors [11] proposed by the World Health Organization (WHO) in 2003. Two pathologists conducted the diagnosis (Figure 1); both pathologists were blinded to the study design and outcomes. The positivity of ER, PR and human epidermal growth factor receptor 2 (HER2) was determined according to the Chinese Guidelines for Immunohistochemical Detection of Estrogen and Progesterone Receptors in Breast Cancer (version 2015) and the Guidelines for HER2 Detection in Breast Cancer (version 14).
Follow-up
A total of 104 patients with IMPC were followed up for a median of 34 months. Follow-up analyses were conducted utilizing inpatient or outpatient medical records, telephonic surveys, and WeChat records. The follow-up initiation time was the date of the operation and concluded on December 31, 2020. The endpoints included disease progression, death, missing visits, or follow-up deadlines. Disease-free survival (DFS) was defined as the time from the postoperative period until the occurrence of the first local recurrence, distant metastasis, or death. Figure 1 illustrates the study design.
Statistical analysis
IBM SPSS Statistics (Armonk, NY), version 25 was used in statistical analyses. The χ2 test was employed for comparison. The survival curve was plotted by the Kaplan-Meier method, and the log-rank test was performed for univariate analyses. Cox regression multivariate analysis was used to investigate the factors affecting the prognosis of IMPC. P < 0.05 was deemed statistically significant. The propensity score method is a statistical technique used to reduce selection bias in observational study samples to equate the distribution of confounding covariates, such as micropapillary features, endocrine therapy, lymphatic invasion, ER-positive expression rate, and molecular subtypes between the IMPC and NSIBC groups. Propensity score matching was executed using SPSS statistical software version 23 to find qualified participants.
Results
Constituent ratio of IMPC patients
A total of 4,532 breast cancer patients were admitted to the hospital. Among them, 227 patients were diagnosed with IMPC, accounting for 5.01% of the total. Of these IMPC cases, 83.70% (37/227) had micropapillary features combined with other pathological types. The IMPC constituent ratio increased progressively from 1.12% (9/807) in 2015, to 4.28% (36/841) in 2016, 5.61% (50/891) in 2017, 6.21% (61/983) in 2018, and 7.03% (71/1010) in 2019.
Clinicopathological features of IMPC and NSIBC
Statistically significant differences were observed in the proportion of endocrine therapy, lymphatic invasion, ER-positive expression rate, and molecular subtypes between the IMPC and NSIBC groups (p < 0.05). Furthermore, no significant difference was found in age, treatment method, T stage, N stage, histological grade, vascular invasion, TNM stage, PR-positive rate, Ki-67 expression, or HER2-positive rate between the two groups (p > 0.05) (Tables I and II).
Table I
Clinical features of patients with IMPC and NSIBC during 2015–2019
Table II
Pathological features of patients with IMPC and NSIBC during 2015–2019
Survival analysis of patients with IMPC and NSIBC
A total of 104 patients with IMPC were followed up for a median of 34 months. During the follow-up, 9 patients experienced recurrence and metastasis, 6 patients had local recurrence, and 3 patients had distant metastasis. Among the 230 patients with NSIBC, 21 patients experienced recurrence and metastasis, 7 patients had local recurrence, and 14 patients had distant metastasis.
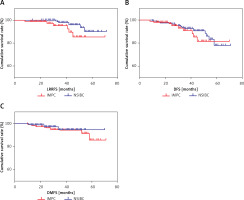
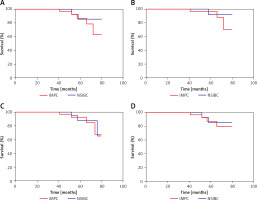
The 5-year loco-regional recurrence-free survival (LRRFS) rate for patients with IMPC and NSIBC was 85.5% and 90.4%, respectively, with a statistically significant difference (p = 0.044). The 5-year DFS rate was 81.5% and 78.0% for IMPC and NSIBC, respectively, without a significant difference (p = 0.385). The 5-year distant metastasis-free survival (DMFS) rate for IMPC and NSIBC was 94.8% and 85.5%, respectively, without a significant difference (p = 0.568) (Figure 2). Figure 3 shows the Kaplan-Meier curves of various statistical parameters of IMPC and NSIBC.
Analysis of factors influencing the prognosis of IMPC through Cox regression
As shown in Table III, Cox regression analysis was performed. The log-rank test results revealed that T stage, N stage, lymphatic invasion, vascular invasion, and ER-positive rate were risk factors for a poor prognosis among patients with IMPC (p < 0.05). Factors that were statistically significant in the univariate analysis were subjected to multivariate analysis. The results of multivariate Cox analysis demonstrated that lymphatic invasion was an independent prognostic factor that affects the survival of patients with IMPC (p < 0.05) (Table III).
Table III
Analysis of factors influencing the prognosis of IMPC using univariate and multivariate Cox regression
Discussion
The epidemiological data released in 2020 showed that breast cancer had overtaken lung cancer as the most common malignancy worldwide, seriously threatening female health. IMPC, a rare breast cancer type, is defined as a subtype of invasive breast cancer in epithelial tumors after two revisions in the Breast Cancer Organization Classification proposed by the WHO [12]. The understanding and development of IMPC in China are slower than those in Western countries [13]. Patients with IMPC showed a higher proportion of lymphatic and vascular invasions [14] and poorer prognosis than patients with NSIBC, thus attracting much attention in the field of breast neoplasms. Due to its low incidence, relevant studies have not reached a consensus on its pathological features, clinical features, treatment methods, and prognosis, and even some of the available data are contradictory. Therefore, we retrospectively analyzed 227 of 4532 patients with invasive breast cancer diagnosed at our hospital from January 2015 to December 2019, and 104 patients with IMPC who met the inclusion criteria were enrolled in the experimental group. A total of 230 patients with NSIBC in the same stage were included in the control group by the propensity score-matched method to eliminate retrospective analysis bias. We compared the clinicopathological features, treatment, and outcomes of the two groups. The results showed that the number of patients with IMPC increased from 1.12% in 2015 to 7.03% in 2019, suggesting a decreased percentage of missed diagnoses at our hospital with an improvement in diagnostic criteria and the pathologists’ understanding of IMPC, similar to those reported previously (3–8%) [15–17]. Studies reported that 0.9–2% of IMPC were homozygous [18, 19]. The present study showed the same findings, with 2.88% of IMPC homozygous and 97.12% of IMPC micropapillary carcinoma with other pathological types (i.e., the mixed type). Therefore, the pathological report indicated an IMPC component and described the percentage of the IMPC component, the receptor status of each histological type, and the degree of lymphatic vessel invasion to guide individualized clinical treatment and prognosis determination.
In this study, the size of an IMPC tumor was mainly T1-2, with lymphatic and vascular invasions. Additionally, AJCC stages I-II and III were 71.15% and 28.85%, respectively, which were consistent with previous study findings. Here, no difference in the histological grade between IMPC and NSIBC was found, which was different from previous studies [20]. Thus, more patients should be evaluated for further verification. Moreover, no significant difference was observed in age, menstrual status, tumor location, surgical method, radiotherapy, targeted therapy, Ki-67 expression, or HER2-positive rate. However, the positive rate of ER in the IMPC group was as high as 79.81%, and the molecular subtype was mainly the luminal type. Hence, the proportion of endocrine therapy was higher, consistent with the results reported by Lewis et al. [21].
Whether the prognosis of patients with IMPC is worse than that of patients with NSIBC remains controversial. The 5-year LRRFS of patients with IMPC in this group was 85.5%, which was significantly different from that of patients with NSIBC and was related to lymphatic and vascular invasions. The 5-year DFS and 5-year DMFS of patients with IMPC were 81.5% and 94.8%, respectively, which were not significantly different from those of patients with NSIBC. This might be because the positive rate of ER was high, the molecular subtype was mainly the luminal type, and patients with IMPC received endocrine therapy. The univariate logistic analysis results showed that T stage, N stage, lymphatic invasion, vascular invasion, and ER-positive rate were risk factors for a poor prognosis of patients with IMPC. The multivariate logistic analysis results showed that lymphatic invasion was an independent prognostic factor affecting the survival of patients with IMPC (p < 0.05).
There are some limitations to this study. First, this is a retrospective study, which may be prone to bias and misclassification, and thus a prospective study would be helpful to vigorously verify these results. Moreover, this is a single institutional study with a small sample size; a future study with a larger cohort from multiple institutions would be helpful to draw stronger conclusions.
Given the higher positive rates of ER and a greater proportion of the luminal type compared to those with NSIBC, it would be interesting to study the patients with these tumor characteristics and compare after implementing these therapeutic modifications. It would help to determine whether changing these therapeutic approaches would have any beneficial effects.
In conclusion, IMPC is a rare and independent pathological type of breast cancer. The diagnostic rate of IMPC may continuously increase in the future with the attention and research of pathologists and clinicians. Patients with IMPC had higher positive rates of ER and a greater proportion of the luminal type compared to those with NSIBC, indicating that endocrine therapy could be strengthened. Although there was no significant difference between 5-year DFS and DMFS, the 5-year LRRFS was poor, revealing a high loco-regional recurrence rate in IMPC patients. Therefore, local radiotherapy and follow-up should be strengthened after surgery. T stage, N stage, lymphatic invasion, vascular invasion, and ER-positive rate were risk factors for a poor prognosis of IMPC patients. Lymphatic invasion and N stage were independent risk factors affecting the prognosis of IMPC patients. This study offers a reference for the precise and individualized clinical treatment of IMPC patients.