Introduction
Androgenetic alopecia (AGA) or male pattern baldness is defined as hair loss with characteristic patterns. The incidence and prevalence of this disease are variable, depending on age and race, and it is more prevalent in the Caucasian population [1, 2]. Alopecia is the most prevalent manifestation. Although the data are contradictory, it is estimated that up to 30% of white males will suffer this type of alopecia in their thirties, reaching 50% for males in their fifties [3, 4]. This disease is of great importance due to the psychosocial problems that it causes in patients, such as severe symptoms of anxiety and depression [5, 6]. AGA is a polygenic disease that gradually shortens the duration of the anagen phase with an increase in the telogen phase. This causes miniaturization of the follicles [7, 8]. In this way, the new hair becomes shorter, which finally leads to the visual appearance of baldness [9]. Numerous studies have established that androgens play a major role in this condition and are therefore determinants of its development and progression [10–12].
It is thought that the tissue changes that occur in alopecia are driven by androgens, but most of the molecular mechanisms involved are unknown, resulting in challenges regarding the treatment of these patients. Research has focused on hair follicles (HFs) for studying the behaviour of multipotent stem cells, highlighting the role of the epidermis (EP), the hair bulb and the sebaceous glands in the repopulation of the EP after injury [13, 14]. A recent study describes how there is an alteration of the stem cell markers in the different structures of the scalp [15]. To date, the best marker for stem cells in human hair is cytokeratin 15 (CK15). Some studies have highlighted the importance of CK15 in some types of alopecia and the change in its expression profile in HFs [16, 17]. On the other hand, Mahalingam [18] reported the lack of specificity of cytokeratin-15 loss in scarring alopecias. This point is very important for understanding the role of cytokeratin-15 in all alopecia types.
One of the facts that has been observed in AGA is the change in cellular homeostasis of the affected tissue, manifesting as a state of cellular senescence [19]. Within cellular senescence, one of the cellular markers that is altered in dermatological disease is p15/16 [20, 21]. Furthermore, some authors have highlighted the importance of the extracellular matrix, especially elastic fibres [22, 23]. Overall, a better understanding of the physiopathological aspects of the disease would help in the development of possible therapeutic targets.
Different studies have shown the importance of the extracellular matrix and cytokeratin 15 in cellular viability in dermatological diseases, specifically in alopecia with special focus on AGA. AGA is a chronic disease with a high prevalence. It is necessary to know the possible changes that can occur in the HF and its nearby appendages. The purpose of this study was to determine the changes in the protein expression patterns of cytokeratin 15, tropoelastin and the senescence markers p15/16 in the tissue of patients with AGA.
Material and methods
Study population
An observational, analytical and prospective cohort study was performed in which 57 patients diagnosed with AGA were analysed. Having signed an informed consent form, a clinical history was compiled for each participant, and a general physical examination was performed. The inclusion criteria were patients diagnosed with AGA type III, based on the Ebling Classification for men. The exclusion criteria were patients with scarring alopecia, hypotrichosis, telogen effluvium, alopecia areata, and anagen effluvium. The mean age of the patients was 42.31 ±3.01 years.
Ethics and consent to participate
This study was carried out in accordance with the basic ethical principles – autonomy, beneficence, non-maleficence and distributive justice – and its development followed the rules of Good Clinical Practice, the principles set forth in the last Declaration of Helsinki (2013) and the Convention from Oviedo (1997). All pathological, clinical or personal data were anonymized and separated from any personal identifiers. The patient was duly informed, and informed consent of each subject was requested in writing.
Obtaining and processing the samples
Two tissue biopsies were taken from the same patient with a 6-mm punch: 1 from a control area in which there were healthy scalp follicles that were clinically and microscopically normal (generally occipital) (area C) and 1 from the area affected by alopecia (area A). These fragments were each placed in a sterile tube containing MEM (Minimum Essential Medium) with 1% antibiotic/antimycotic (both from Thermo Fisher Scientific, Waltham, MA, USA). The transfer and processing of the tissue samples were performed in all cases within 6 h after collection.
In the laboratory, the samples were processed in a class II Telstar AV 30/70 Müller 220 V 50 MHz laminar flow hood (Telstar SA Group, Terrassa, Spain), ensuring a sterile environment. The samples conserved in MEM were used for histological studies and cell isolation for in vitro studies. The samples were washed/hydrated several times with MEM without antibiotic to remove blood cells and were cut into fragments that were stored in F13 (60% ethanol, 20% methanol, 7% polyethylene glycol, and 13% distilled H2O).
Cell isolation and culture
Under sterile conditions, the tissue segments were washed several times with MEM under sterile conditions and then opened longitudinally. After removing the adipose tissue, the sample was cut into small explants (1 mm2). Subsequently, the explants were digested in 0.1% type I collagenase (Worthington) in MEM (1 h at 37°C) under constant stirring. The enzyme reaction was stopped by adding the same volume of culture medium; then, the solution was centrifuged at 200 g for 7 min, and the medium was discarded. These explants were placed in 25 cm2 Roux flasks (Nyclon-Intermed; Nunc A/S, Roskildo, Denmark) in which 0.5 ml of Amniomax complete medium (Gibco BRL, Life Technologies Carlsbad, CA, USA) was added to maintain moisture on the cultivation surface and to improve explant adherence. The culture flasks were then incubated vertically at 37°C in a 5% CO2 environment in a culture oven for 2 h. Then, 2.5 ml of Amniomax was added to the flasks, and the flasks were incubated horizontally under the above conditions. Care was taken to avoid movements that could cause the explant to detach. The culture medium was carefully replaced twice a week, following established protocols for primary cultures [24].
Once the cells had grown to confluence, the cells were subcultured. This involved removing the medium and rinsing the cells 3 times in 2 ml of Hank’s balanced salt solution (Gibco BRL, Life Technologies), followed by the addition of 2 ml of trypsin-ethylenediaminetetraacetic acid solution at 1 : 250 (Gibco BRL, Life Technologies) and incubation at 37°C for 5 min. The enzymatic reaction was stopped by adding 4 ml of culture medium. The resulting cell suspension was centrifuged at 200 g for 7 min, and the cell pellet was resuspended in 9 ml of Amniomax medium. The cells in suspension were cultured at a density of 3 ml per 25 cm2 Roux flask until confluence was obtained in an incubator with an atmosphere humidified with 5% CO2 at 37°C. The cells were maintained for 90 days to observe their viability.
Histological studies
After fixation, the samples were dehydrated and embedded, as follows. The tissue was washed with distilled water for 20 min and then dehydrated by serial incubations in an ascending alcohol gradient, followed by standardized paraffin-embedding protocols for subsequent histopathological study [25]. After dehydration, paraffin blocks were made using moulds. Once the paraffin was solidified, an HM 350 S rotation microtome (Thermo Fisher Scientific, Massachusetts, USA) was used to obtain 5 µm-thick sections, which were spread in a hot water bath and collected on glass slides treated with a solution of 10% polylysine to facilitate the adhesion of the sections.
Protein expression studies
Protein expression analysis was performed by detecting antigen-antibody reactions (avidin-biotin complex) using chromogen peroxidase and phosphatase according to the following protocol. 1. The samples were washed 3 times with 1× phosphate-buffered saline (PBS) for 5 min each wash. 2. Non-specific binding sites were blocked with 3% bovine serum albumin (BSA) in PBS for 30 min at room temperature. 3. The samples were incubated with primary antibody diluted in 3% BSA and PBS overnight at 4°C (Cytokeratin 15: Abcam (AC-0018), dilution 1 : 400, 100% Triton 0.1% in PBS, 10 min, before incubation with blocking solution; Tropoelastin: Dr. Mecham (Washington University, EEUU), dilution: 1 : 750; p15/p16: Santa Cruz Biotechnology (sc-377412), dilution 1 : 50, 10 mM sodium citrate pH = 6 before incubation with blocking solution). 4. The samples were rinsed in PBS 3 times for 5 min each wash. 5. The samples were incubated with biotin-conjugated secondary antibody diluted in PBS for 1.5 h at room temperature (IgG (Rabbit) Sigma-Aldrich (B5283), dilution 1 : 1000; IgG (Mouse) Sigma-Aldrich (B0529), dilution 1 : 300). 6. The samples were washed with PBS 3 times for 5 min each wash. 7. To detect tropoelastin and p15/16, the samples were incubated with the avidin-peroxidase conjugate ExtrAvidin-Peroxidase (Sigma-Aldrich, St. Louis, MO, USA) for 60 min at room temperature. To detect CK15, the samples were incubated with an avidin-phosphatase conjugate (ExtrAvidin-Alkaline Phosphatase, Sigma-Aldrich, St. Louis, MO, USA) under the same conditions. 8. The samples were washed in PBS 3 times for 5 min each wash. 9. To visualize tropoelastin and p15/16, samples were incubated in the chromogenic substrate diaminobenzidine (Kit DAB, SK-4100) (Vector Laboratories, Burlingame, CA, USA). The chromogenic substrate was prepared immediately before exposure (5 ml of distilled water, 2 drops of buffer, 4 drops of DAB, and 2 drops of hydrogen peroxide). This technique results in brown staining. To visualize CK15, samples were incubated in alkaline chromogenic substrate for 15 min. 10. The samples were washed with distilled water 3 times for 5 min each wash to stop the reaction. 11. For nuclear staining, the samples were incubated in Carazzi’s haematoxylin for 5–15 min. 12. The samples were then rinsed in running water for 10 min and mounted in aqueous medium (Plasdone).
For all immunohistochemical studies, sections of the same tissue were used as a negative control, in which the incubation with primary antibody was substituted with incubation in blocking solution.
Statistical analysis
For each of the patients in the established groups, 5 sections and 10 fields per section were randomly selected. Patients were described as positive when the marked mean area in the analysed sample was greater than or equal to 5% of the total, following the anatomopathological protocol of Remmele and Schicketanz [26] and Cristóbal et al. [27]. The preparations were examined under a Zeiss Axiophot optical microscope (Carl Zeiss, Germany) equipped with an AxioCam HRc digital camera (Carl Zeiss, Germany). For the statistical analysis, GraphPad Prism 6.0 was used. The Mann-Whitney U test was applied. Data are expressed as the mean ± standard error of the mean (SEM). Significance was established at a value of p < 0.05 (*).
Results
Cell viability
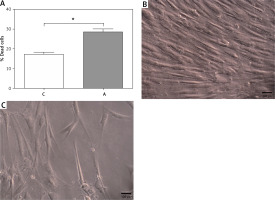
In vitro studies of fibroblasts from the scalp of patients showed differential behaviour with respect to their viability in cell culture conditions. It was observed that cells from areas with alopecia (A) showed a significantly higher percentage of dead cells after 90 days (17.00 ±1.09% C vs. 28.50 ±1.41% A, *p < 0.05). The cells from the control area (C) showed long-term cell viability characterized by the preservation of cell morphology and increased multilayer growth. These characteristics were not observed in area A, where cells grew in a monolayer (Figure 1).
Cytokeratin 15 expression
The study of CK15 protein expression by immunohistochemical techniques showed significant differences in relation to its expression in different parts of the HF and other skin appendages in areas C and A.
CK15 protein expression was significantly lower in the HF of area A (area A: 0.73 ±0.13; area C: 1.96 ±0.23; *p < 0.05) (Figure 2). For the hair bulb (HB), no differences in CK15 expression were observed between the 2 areas (0.41 ±0.04 C vs. 0.32 ±0.04 A). In the sebaceous gland (SG), CK15 expression was significantly lower in area A compared to area C (1.87 ±0.32 C vs. 0.31 ±0.09 A, *p < 0.05) (Figure 2).
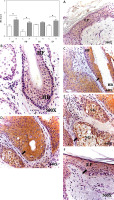
Figure 2
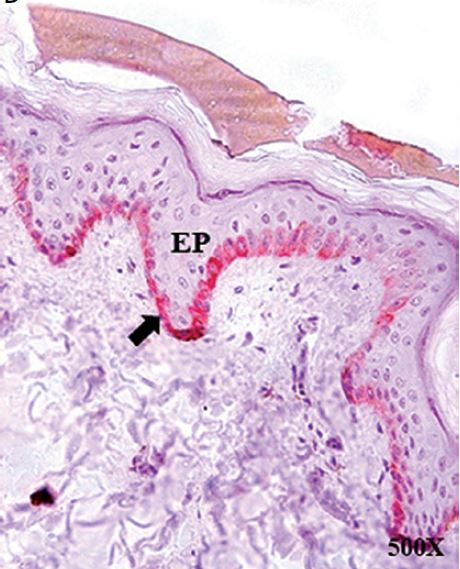
Protein expression of cytokeratin 15 (CK15) in the hair follicle (HF), hair bulb (HB), sebaceous gland (SG) and epidermis (EP) in the control area (C) and in the area with alopecia (A). p < 0.05 (*). Protein expression images of CK15 in the HF and HB of the control area (A–C) and area with alopecia (E), as well as in the EP of area C (D) and A (F). Arrow: immunoprecipitated
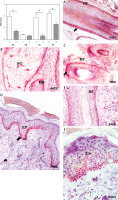
The EP of the area near the outer root sheath was studied, and CK15 protein expression in area A was significantly lower (2.25 ±1.29 C vs. 1.29 ±0.11, *p < 0.05). The histological study revealed that the lower CK15 expression in the EP of area A appeared as small accumulations in the deeper epidermal region (Figure 2). No expression greater than 5% was observed in the dermis of either area and thus was considered negative expression.
Tropoelastin expression
Immunohistochemical techniques were used to study TE protein expression in the HF as well as other skin appendages. The histological analysis revealed differences in expression not only between areas C and A but also between different tissue structures (HF, HB, SG and EP). TE protein expression in the HF of area A, compared with that in the HF of area C, was significantly lower (0.96 ±0.16 C vs. 0.46 ±0.08 A, *p < 0.05) (Figure 3). For the HB, no significant differences were observed in terms of protein expression between the 2 study groups (0.27 ±0.09 C vs. 0.15 ±0.06 A). The mean TE expression in the HB was the lowest of all the structures studied. TE expression in the SG of area A was significantly lower (0.75 ±0.09 C vs. 0.18 ±0.05 A, *p < 0.05), as was TE expression in the EP of area A (1.27 ±0.16 C vs. 0.79 ±0.11 A, *p < 0.05) (Figure 3). TE expression was observed in the papillary dermis, with a mean of 0.81 ±0.12 in area C. Area A did not show expression greater than 5%; therefore, its expression was considered negative (Figure 3).
Figure 3
Protein expression of tropoelastin (TE) in the hair follicle (HF), hair bulb (HB), sebaceous gland (SG) and epidermis (EP) in the control area (C) and in the area with alopecia (A). p < 0.05 (*) Protein expression images of TE in the HF and HB of the control area (A, B) and in the affected area (D, E), as well as in the EP of area C (C) and A (F). Arrow: immunoprecipitated
Expression of cellular senescence markers
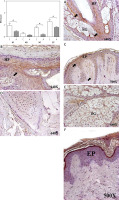
The study of cellular senescence marker protein expression was performed via the evaluation of p15/p16. p15/p16 protein expression in area A increased as a function of the different parts. The score was significantly higher in the HF of area A (0.854 ±0.095 C vs. 1.396 ±0.183 A, *p < 0.05); similarly, significantly higher p15/p16 protein expression was observed in HB (0.396 ±0.125 C vs. 1.115 ±0.143 A, *p < 0.05) and in EP (0.860 ±0.129 C vs. 1.292 ±0.137 A, *p < 0.05) (Figure 4). No increase in the p15/p16 cellular senescence marker expression was observed in the SG of these patients (0.979 ±0.048 C vs. 1.146 ±0.078 A).
Figure 4
Protein expression of senescence markers (p15/p16) in the hair follicle (HF), hair bulb (HB), sebaceous gland (SG) and epidermis (EP) in the control area (C) and in the area with alopecia (A). p < 0.05 (*) Protein expression images of p15/ p16 in the HF and HB of the control area (B) and in the affected area (C–E), as well as in the EP in area C (A) and A (F). Arrow: immunoprecipitated
Discussion
The different mechanisms involved in skin tissue remodelling in AGA are being slowly revealed. Most of the current research focuses on studying the mechanisms in scarring alopecia and alopecia areata, due to its repercussions on the quality of life of patients [17, 28, 29]. Stem cells play a major role in remodelling and maintaining the structure and viability of the HFs, making it a key point for the development of potential therapies [30, 31]. Although some authors have suggested that the HB provides a niche for stem cells, most studies show that the biochemical characteristics and the pattern of gene expression are not well defined for these cells [14, 32, 33].
Our results showed no significant differences in CK15 and TE in the HB, observing very limited expression in the control and alopecic areas, but positive p15/p16 expression. However, TE in the extracellular matrix near the HB was strongly expressed in the control area, decreasing in the alopecic area. These findings could suggest the effect that the extracellular matrix could have on the viability of the bulb itself and HFs. Similarly, numerous studies have demonstrated the implication of decreased expression of the different mechanisms involved in the assembly of TE in dermatological diseases [22, 34]. The collagen-elastic fibres in the tissues give extensibility and strength to the skin and major vessels; it is crucial that the fibres are properly assembled from microfibrillar components secreted from fibroblasts such as soluble TE monomers [35]. Our results showed that the cellular viability of the fibroblasts in the alopecic area decreased significantly in the long term; therefore, the decrease may be related to the lower protein expression observed in these patients. The decrease in cell viability in the short and long term has been related by some authors to processes of cellular senescence and changes in the expression profile of markers involved in different cell transduction pathways [22, 36, 37]. Upton et al. [19] reported that patients with AGA presented an increase in cellular senescence. This process could be related to the lower regenerative capacity of this part of the tissue, as noted by numerous authors, as it is related to a cellular ageing process [38]. Therefore, future studies should be directed towards the study of the possible implications of senescence and the disruption of cell communication in these patients. The functioning of elastic fibres has been related to CK15; the expression of both could be jointly disrupted in human skin disorders [39, 40].
CK15 is related to different types of alopecia, and its expression profile can change in different components of the scalp, such as the HF [14, 17, 41]. Our results showed that CK15 decreased significantly in the HF of tissue affected by AGA; in this same tissue, a significant decrease in the SG and EP was also observed. The SG is described by authors as primordial in the regeneration of skin tissue, and its disruption can have important consequences [42–44]. Sinclair et al. [45] described how alterations in the sebaceous gland affect alterations of the hair follicle in patients with AGA. In addition, these authors described how the hair follicle musculature was affected in these patients [46]. In this context, our results showed that the SG shows less protein expression than the previously described CK15 and tropoelastin, suggesting involvement of the SG in the tissue affected by AGA, with consequences for its regeneration.
Another important aspect is the EP itself. Piérard-Franchimont and Piérard [47] related the disrupted functionality of the EP with alopecia areata, as well as SG modifications. Current studies have shown how the EP in patients with alopecia can undergo changes in protein expression profiles, affecting normal physiology and regeneration [48, 49]. The present study showed how CK15 and TE protein expression was modified in the EP of areas with AGA, suggesting that there is involvement of different components of scalp tissue with alopecia, with consequences for cellular homeostasis. One of the key points that determine tissue effectiveness in this disease is vascularization. Lachgar et al. [50] demonstrated how the effect of vasodilators in patients with AGA had positive effects on the regeneration of follicles with greater expression of VEGF. In in vitro studies, the best vascularization allowed higher survival of the cells of patients with AGA [51]. Poor vascularization of tissues leads to hypoxia processes that can lead to tissue damage and death, or adaptive physiological processes to avoid this damage such as cellular senescence [52–54].
The obtained results showed that AGA scalp tissue undergoes damage that affects different components of follicular stem cells, as well as the extracellular matrix itself, with decreased CK15 and TE protein expression and increased protein expression of cellular senescence markers such as p15/p16. The obtained results showed how the extracellular matrix undergoes a change that could be related to alterations in cell processes. Patients with AGA undergo changes in the expression of elastic fibres with consequences for cell homeostasis. This point is important; histopathological studies can help focus the way of treating the patient. Knowing what the state of hair tissue is can be decisive in therapies such as hair transplant procedures or other processes. One of the limitations of this study is the inability to observe the behaviour of these proteins in healthy people. Future studies should implement as far as possible tissue engineering in AGA patients and in healthy controls for a better understanding. Such studies should aim to understand the set of interrelated mechanisms that modulate the follicular stem cells themselves and neighbouring elastic fibres. This will allow the development of treatment targets and thus more effective and selective therapeutic approaches to AGA.