Icosapent ethyl is a highly purified omega 3 fatty acid known as eicosapentaenoic acid (EPA). It has been shown to reduce triglyceride levels, and is used in conjunction with dietary and lifestyle measures and other cardiovascular drugs to reduce the risk of adverse cardiovascular events. The Japan EPA Lipid Intervention Study (JELIS) reported that among 18,645 Japanese patients with hypercholesterolaemia, treatment with 1.8 g of EPA daily on top of low-intensity statin therapy resulted in a significant, 19% reduction in the risk of major coronary events compared to patients receiving statin therapy alone [1]. More recently, the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) reported a significant reduction in major ischemic events, including cardiovascular death, in statin-treated patients with elevated triglycerides receiving 2 g of icosapent ethyl twice daily compared to placebo [2]. Furthermore, the number needed to treat to avoid one primary end-point event was 21 (95% CI, 15 to 33) over a median follow-up of 4.9 years [2]. As a result, icosapent ethyl was recently approved in Europe to reduce cardiovascular risk in statin-treated adults with elevated triglycerides (≥ 150 mg/dl (≥ 1.7 mmol/l)) and other high-risk features, in line with the population characteristics in the REDUCE-IT study. The positive outcomes observed with EPA in these two studies contrast with previous, less favourable results in terms of cardiovascular risk reduction with other n-3 fatty acid formulations [3]. Nevertheless, this innovative treatment has just been approved in France for the treatment of patients with atherosclerotic cardiovascular disease, but data about the clinical profiles of coronary artery disease (CAD) patients eligible for icosapent ethyl are lacking. Therefore, we used data from the recent national ODIACOR registry to determine the number of patients potentially eligible for icosapent ethyl treatment among a representative sample of CAD patients in daily practice.
Methods
This prospective national database analysis included consecutive patients with documented type 2 diabetes (T2D) and CAD consulting private practice cardiologists registered with the National College of French Cardiologists (CNCF). Consecutive patients consulting between March 2019 and December 2020 were eligible for inclusion in the registry. In accordance with current regulations, this registry was declared to the Institut Nationale des Données de Santé (the national health data institute). Data were collected on a dedicated website managed by the CNCF, using an electronic case report form (eCRF). The eCRF recorded: (1) patient data, namely: clinical characteristics, date of consultation; (2) healthcare provider data, namely: occupation, specialty, place of practice; (3) claims data from private clinics, namely: drugs prescribed, medical procedures performed, biological procedures prescribed, medical devices used, date of examinations/procedures; (4) hospitalization data, namely: date of index event, coronary revascularisation, recurrent cardiovascular events.
Results
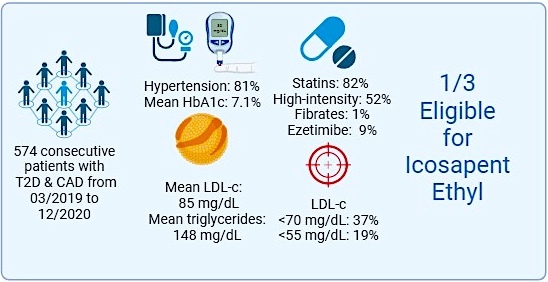
Among a total of 574 patients with CAD and T2D included in the analysis, the mean age was 71 years, and the majority (81%) were male, with a high prevalence of other risk factors (81% with hypertension) and numerous cardiovascular comorbidities, e.g. history of myocardial infarction in 47%, coronary revascularization in 78% and peripheral arterial disease in 16%. Biological data of the study population showed a mean triglyceride level of 148 mg/dl, serum total cholesterol of 156 mg/dl, lipid-density lipoprotein (LDL) cholesterol of 85 mg/dl, and a mean glycated haemoglobin of 7.1%. A total of 82% of patients received statins, with only 52% receiving high-intensity statins. Fibrates were prescribed in 1% of the population and ezetimibe only in 9% of cases despite the guidelines, as only 37% had LDL cholesterol < 70 mg/dl, and 19% < 55 mg/dl.
According to the criteria of the National Lipid Association, 31% of these routine CAD patients with T2D would be eligible for treatment with icosapent ethyl at a total dose of 4 g/day. Our data indicated high therapeutic inertia, since lipid-lowering therapy was mostly not intensified (in only < 2% of cases), and more frequently, the intensity of treatment was reduced (intensity decreased in 21% compared to treatment prescribed at the index event).
Discussion
This contemporary survey highlights the considerable margin for improvement that remains in terms of lipid-lowering management. Indeed, we observed that only about one half of CAD patients with diabetes were treated with high-intensity statins, and only 9% were prescribed a combination with ezetimibe. Therefore, the LDL-C goals recommended by professional societies [4, 5] were rarely obtained in this high-risk population, leaving considerable numbers of patients undertreated and exposed to a persistent risk of adverse cardiac events. In France, PCSK9 inhibitors may only be prescribed in patients with an LDL-C above 70 mg/dl despite being treated with the maximal tolerated dose of statins plus ezetimibe. Accordingly, the rate of use of these new drugs is marginal. The same findings are reported for the anti-diabetic strategy, as GLP1RA were prescribed only in 14% of T2D patients, and SGLT2 inhibitors were not yet available on the French market. Overall, the lipid profiles were suboptimal, and as a result, one third of patients would be eligible for icosapent ethyl, which could provide further clinical benefits by reducing ischemic events. The addition of icosapent ethyl to the therapeutic armamentarium in this situation could offer alternative treatment solutions for physicians, thereby combatting therapeutic inertia, and reducing cardiovascular risk.
Several factors may explain the non-compliance with treatment recommendations observed in this study. These include heterogeneity in the guidelines (the latest guidelines from the French national health authority date from 2004), a lack of knowledge of the most recent ESC guidelines, suboptimal knowledge concerning the use of lipid-lowering therapy combinations, a fear of side-effects with high-intensity statins, and the use of a stepwise strategy with progressively increasing statin doses, but which is often not followed by initiation of ezetimibe [6, 7]. An underestimation of benefits provided by a profound decrease in LDL-C, and suboptimal knowledge of randomized cardiovascular outcome trials testing ezetimibe (IMPROVE-IT) [8] or PCSK9i (FOURIER and ODYSSEY Outcomes) [9, 10] by cardiologists or general practitioners who are not experts in this field, also seem to contribute to the reported non-compliance in our survey.
One potential limitation of our study is that estimates of eligibility for EPA were based on current treatment. Since 18% of patients received no statins, and only 52% received high-intensity statins, the TG levels qualifying patients for EPA eligibility could have been reduced by more intensive statin therapy. If statin and potentially ezetimibe therapy were optimized in this population, the overall eligibility for EPA might actually be lower than estimated.
In conclusion, in this contemporary survey among a population of consecutive, unselected CAD patients with T2D, mostly treated with statins, and with an average triglyceride level of 148 mg/dl, 31% would be potentially eligible to benefit from icosapent ethyl. These data underline that there is a large margin for the addition of this new drug to the preventive armamentarium with a view to further reducing the risk of ischemic events in these patients.
Conflict of interest
P.S. declares that he has received speaker fees from Astra Zeneca, Axis TV, BMS, Les laboratoires Servier, Novartis, Novonordisk, Sanofi, and Vifor, outside the present work. F.E. declares speaker fees from Sanofi and consultant fees from GlaxoSmithKline, outside of the present work. M.B. declares: speakers bureau: Amgen, KRKA, Polpharma, Novartis, Sanofi-Aventis, Teva, Zentiva; consultant to Amgen, Daichii Sankyo, Esperion, Novartis, Sanofi-Aventis; grants from Amgen, Sanofi and Valeant. N.L. declares consultant fees from BMS, Bayer outside the present work. JCD has no conflict of interest to declare.