Introduction
Head traumas (HT) are a regular occurrence for every small child. Their most common causes are falls and collisions with furniture and other objects, or traffic accidents in older children and adolescents [1, 2]. The Glasgow Coma Scale (GCS) is commonly used to assess consciousness after HT [3]. The Children Coma Scale (CCS) in Raimondis’ modification is used in children under 3 years of age [4]. The majority of HT in children are mild, scoring 13–15 on the GCS/CCS, and parents often do not seek medical attention, given the apparent trivial nature of the symptoms [5, 6]. Therefore, the real number of HT cannot be estimated. The National Health Injury Foundation (NHIF) named HT a “silent epidemic” [7]. It is estimated that only 5–15% of patients after HT go to the emergency department (ED) due to alarming symptoms: unconsciousness, balance disorders, vertigo, vomiting or scalp wounds [8, 9]. Most vegetative symptoms resolve within a few days after the trauma with the exception of headaches, which can last for weeks or even months [10, 11].
The routine management of a child after a mild HT is based on hospital observation (2–3 days), ultrasound and/or computed tomography (CT) scans, neurological and ophthalmologic examination [12, 13]. In the physical examination, the body balance tests of Romberg, Unterberger and Babiński-Weil (“star-walk”) are used. There is, however, no simple method of objective balance assessment. The examination on the stabilometric platform proposed by the authors enables an objective, simple and repeatable assessment of the static body balance of these patients [14–16].
The incidence of balance disorders and different types of vertigo in the general population is estimated at approximately 20–30%, while in children at approximately 8–18% [17, 18]. The authors decided to investigate the occurrence of balance disorders in children after mild HT, because of a lack of such reports in the available literature.
Material and methods
Between 2014 and 2016 in the Departments of Pediatric Surgery at the University Pediatric Centre, Central University Hospital, Medical University of Lodz, 375 post-HT patients were hospitalized. 90 patients were enrolled in the study group (SG): 58 boys (64.44%) and 32 girls (35.56%), aged 7–18 years.
The inclusion criteria for the study were: recent mild HT (13–15 GCS points), no scalp wounds or skull fractures, age 7 and over, very good and good body posture according to Kasperczyk. Disease history – chronic diseases and past HT – was the exclusion criterion of the study. The lower age limit in SG was set at 7 years, because at this age children start to maintain body balance at a level similar to adults [19, 20].
Approval for the study was obtained from the Bioethics Committee of the Medical University of Lodz (No. RNN/388/12/KB of May 22, 2012).
Subject characteristics
Biometric data of children from the SG at both time points of the study and the children from the RG did not differ significantly (Table I). There were no differences between the SG (at both time points) and the RG in both sexes (p = 0.845) and age (p = 0.89) in all age subgroups.
Table I
Biometric data of children from the study group and the controls
The reference group (RG) consisted of 50 healthy children aged 7–18 from schools in the Kleszczow community: 30 (60%) boys and 20 (40%) girls.
The patients in the SG and RG were divided into three age subgroups based on developmental age and the age of perfecting balance skills: 7–10, 11–15 and 16–18 years [19–21].
Methods
The study was conducted according to the following protocol:
Personal questionnaire (physical examination); biometric data, disease history – chronic diseases and past HT.
Measurement of the length of the lower limbs (relative length); in a lying position on the back from the upper anterior iliac spine to the medial ankle [22].
Body posture according to Kasperczyk (very good 1–3 points, good 4–6 points, poor > 7 points), consisted of observing individual components of the posture (head, shoulders, chest, spine, back, abdomen and knees) in the frontal and sagittal plane in a free-standing position [23].
Tests on a two-plate stabilometric platform (CQ Elektronik System, Poland).
The platform is built of several tensometric sensors, which enable the measurement of the reaction of ground forces (forces exerted by the feet on the ground) and the variability of the direction of the impact of these forces. In practice, this allows the registration of left and right lower limb action (load and body sway directions) together or separately. Special software evaluates the behavior of the body in space in the form of a projection of the center of gravity on the support plane (center of pressure – COP), creating a graph of COP deflections in the sagittal and frontal plane – a stabilogram [24, 25].
The examination was carried out in a barefoot, quiet standing position, with arms along the torso, with eyes open (EO) and focused on a blank monitor screen (no biofeedback) for 30 s [25]. Seven parameters of the stabilogram were measured and analyzed:
SP-EO (mm) (sway path) – Total length of the COP path (center of pressure of the feet), counted on both axes (X and Y, 2D). The path length is the sum of the distances between the position of the COP point in successive trials.
MA-EO (mm) (mean amplitude) – Average COP deflection (X and Y, 2D) (radius of deflections from the center of the coordinate system – point 0).
MAAP-EO (mm) – Average deflection (radius) of COP from point 0 in the direction of the Y axis (AP sagittal plane, antero-posterior deflection).
MAML-EO (mm) – Average deflection (radius) of COP from point 0 in the direction of the X axis (ML frontal plane, lateral deflection).
MV-EO (mm/s) (mean velocity) – Average speed of movement of the COP point on the XY (2D) axes. This is the quotient of the total length of the COP (SP-EO) sway path and time (30 s trial).
MVAP-EO (mm/s) – Average speed of movement of the COP point on the Y axis (AP sagittal plane, anterolateral deflection).
MVML-EO (mm/s) – Average speed of movement of the COP point on the X axis (ML frontal plane, lateral deflection).
These seven parameters were selected as the most representative for all groups of 137 parameters available for measurements. Each one of these seven represented a group of parameters showing the particular movements and deflections of the body [25].
The examination was performed 48 h after HT and repeated 12 weeks after the trauma. The second examination was reported by 54 out of 90 (60%) qualified patients (35 boys – 64.81% and 19 girls – 35.19%).
Statistical analysis
The comparison of nominal variables was carried out using Pearson’s χ2 test. Intergroup differences were analyzed using Student’s t-test for dependent pairs (a comparison of SG results between two time points of the study) and Student’s t-test for independent pairs (comparisons between SG results after 12 weeks and RG scores). The relation between values of examined parameters and patients’ age was analyzed with ANOVA. Values of p < 0.05 were considered significant. The calculations were performed using the Statistica 12.5 package.
Results
The most frequent causes of HT in the SG were: violence – 28.9% (beatings, intentional pushes), traffic accidents – 26.7% (pedestrians, bicycle and car accidents), accidental falls – 24.4%, and accidents caused by physical activity (playing football, cycling/roller-skating, playing hockey) – 20%.
In the period analyzed, an average of 3,310 patients were hospitalized annually in the surgical departments of the Pediatric Center of the Medical University of Lodz, of which 125 were children with HT (mean: 3.78%, 2.92–4.23%). In the same period of time, an average of 27,303 patients were given medical advice annually in the ED, including 1.805 children with a HT (mean: 6.6%, 6.13–7.37%). The hospitalization rate after HT was 6.93% on average (4.33–9.64%) over the period considered.
Length of lower limbs
Differences in the relative length of the left and right lower limbs in the entire SG did not exceed 0.5 cm and were statistically insignificant.
Body posture according to Kasperczyk
Only children with very good (1–3 points) and good (4–6 points) posture ratings qualified for the posturographic examination.
Posturographic examination
The entire study group
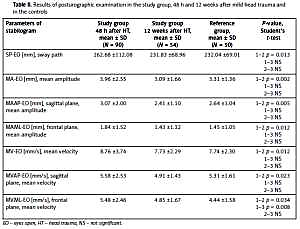
The values of all recorded parameters in the entire SG 48 h after HT were significantly higher than 12 weeks after the injury (p < 0.034). Their values 12 weeks after the injury decreased to the level of the values in the RG (NS – not significant) (Table II).
Table II
Results of posturographic examination in the study group, 48 h and 12 weeks after mild head trauma and in the controls
Age subgroup 7–10 years
The values of all tested parameters in children of the SG aged 7–10 years 48 h after HT were significantly higher than 12 weeks after the injury (p < 0.05) and higher than in the RG (NS). Their values 12 weeks after the injury decreased to the values in the RG (NS).
Age subgroup 11–15 years
The values of all tested parameters in children of the SG aged 11–15 years 48 h after the injury were significantly higher than 12 weeks after HT, and also significantly higher than in the RG. Their values 12 weeks after the injury decreased to the level of RG values (NS) (Table III).
Table III
Results of posturographic examination in study group, 48 h and 12 weeks after head trauma and in the controls in children aged 11–15
Age subgroup 16–18 years old
The values of all parameters examined in adolescents of the SG aged 16–18 years 48 h after HT did not differ from the values 12 weeks after the injury (NS) and in the RG (NS).
An interesting finding was the observation of an inverse relationship between the value of each of the tested parameters and the child’s age, both in the SG and in the RG. The lowest values were observed in the oldest children. The values of all tested parameters in the RG decreased with the age of the children (ANOVA, p < 0.008). In the SG 48 h after the injury, the values of all parameters except for MAAP-EO decreased with the age of patients (ANOVA, p < 0.014). Twelve weeks after the injury, a similar relationship was demonstrated: the values of SP-EO, MV-EO, MVAP-EO and MVML-EO decreased with the age of boys (ANOVA, p < 0.001).
Discussion
Head traumas in children are the most common or the second most common (after musculoskeletal injuries) reason for reporting to Emergency Departments (ED). Head traumas are the leading cause of death and acquired neurological disorders in children. Neurological and psychological sequelae are found in more than 1/3 of patients with moderate or severe head trauma [6, 9].
In our material, children after HT represented only about 3.78% of all patients hospitalized in the surgical wards of our center, which results from the fact that the initial diagnostics and observation of these patients were carried out in the ED. In turn, in the ED in the analyzed period, children after HT represented on average 6.6% of all medical counseling provided, while the rate of hospitalization of patients after HT was only 6.93% on average.
In the US alone, between 500,000 and 700,000 children after HT are admitted to a surgical ED per year, and about 95,000 (15–20%) of them are hospitalized [12]. One in 220 children is reported to the ED for HT; most of them suffer from mild HT [5, 6]. Approximately 10% of cases of HT in children in the US are associated with sports: horse riding, ice skating, sledding and cycling, less often football and martial arts [13]. Data from Canadian EDs show that 1 out of 70 visits are patients with HT. Brain injuries are the most common cause of death due to HT (about 25,000 children per year) [26].
In European Union countries, falls (children and the elderly) and traffic accidents (youth and young adults) are the most common cause of HT [27, 28]. Data collected in North America, Europe, Australia and New Zealand show that the annual incidence of HT in children and adolescents aged 0 to 20 years is 691 per 100,000 people reporting to EDs, 74 per 100,000 hospitalized, and 9 per 100,000 dying due to the consequences of HT. According to these authors, the leading cause of HT in children under the age of 5 are falls, and in youth over the age of 15 are traffic accidents. Head trauma caused disability in 20% of the patients hospitalized [9].
The available reports show that in Poland, about 30% of children after HT are hospitalized in surgical wards [29]. In one of the pediatric centers the percentage of children after HT among all patients hospitalized was as high as 67% [30].
The values of the tested parameters and their dispersion in children examined 48 h after HT clearly indicate deterioration of body balance control as a result of the head injury. Thus, the influence of even a mild HT on the postural control in children, especially at the age of 11–15, was visible.
The average speed of deflections (MV-EO), higher values of which we observed 48 h after HT, testifies to the high dynamics of movements performed to maintain the body balance. If the child is calm, the parameter values are lower, while its high values indicate a sudden recovery of balance, i.e. standing less stable.
Standard deviation (SD) values for path length (SP-EO) and average speed (MV-EO in the youngest (7–10 years) and the oldest children (16–18 years) were lower than in children aged 11–15. The greater diversity of the middle group in terms of balance control may be associated with ongoing puberty and emotional maturation in children of this age. A similar relationship can be observed in the results 12 weeks after injury. Children from the middle age group (11–15 years) proved to be the most susceptible to the effects of mild HT. The values of all tested parameters in this group were significantly higher than the values in the RG (Table III). In the youngest group, the values were also higher than in the RG, but the differences were not statistically significant, while in the oldest group, the values did not differ from those in the RG both 48 h and 3 months after HT.
There are only a few reports on the occurrence of balance disorders and vertigo in children after HT in the literature. Rochefort et al. observed an increase of SP-EO, MV-EO, MVAP-EO and MVML-EO in children after concussion in comparison with healthy peers. These authors concluded that 1 month is not enough time for children after HT to again attain full body balance control [31].
The available reports on body balance assessment concern adolescents and adults after concussion due to sport activities. However, these studies were performed using a subjective diagnostic method, i.e. BESS (Balance Error Scoring System), and the results can hardly be compared with the results of the present study [32, 33].
Higher values of COP in the sagittal plane and MAAP-EO radius observed in our patients are consistent with Winter’s hypothesis [34] that the child’s nervous system more intensively controls the vertical body posture in the sagittal plane (reverse pendulum theory) using the ankle strategy. The examined children maintained body balance by “rocking” back and forth, and slightly less so sideways. In fact MAAP-EO was the only parameter which did not decrease significantly with the age of patients 48 h after HT. On the other hand, older people show greater deflections of the COP in the frontal plane, which is related to the balance control using the hip joint strategy and swaying from one leg to the other [34, 35].
Wolff et al. also observed significant decrease of the discussed parameters with age in almost 100 examined children [36]. In contrast, Lebiedowska and Syczewska did not observe any significant differences in the range of amplitude parameters examining without biofeedback healthy children aged 7–18 [37]. Linder et al. examined 6,762 athletes using the Cleveland Clinic-Postural Stability Index (CC-PSI) and observed worse postural stability in youth athletes (age 5–13), especially male, compared to high school (age 14–18) and collegiate athletes (age 19–23). In their opinion optimal concussion management should use objective age- and sex-specific values in the evaluation of postural stability [38]. Similarly, Hugentobler et al. found age a critical and sex not an important factor in the postconcussion postural control assessment of 71 adolescents at the mean age of 14 years [39].
Hay et al. [21] stated that the greatest development of body stabilization skills is up to 10 years of age and is manifested by a decrease in the values of the stabilogram parameters. The results of our research indicate that children aged 7–15 are still improving control of balance and apparently react to factors disturbing homeostasis, e.g. head trauma. It is only after the maturing period (16 and over) that the body’s stability appears to be well fixed and not affected by mild HT.
In the posturographic examination performed by authors in children after mild HT, the best indicators describing disorders and normalization of body balance control processes proved to be the total path length of the COP (SP-EO), the average COP deflection (MA-EO) and the average COP sagittal deflection (MAAP-EO).
In conclusion, posturography should be included in the routine assessment of children after mild head trauma, because this type of injury clearly, though temporarily, disturbs postural control of the body, especially affecting children aged 11–15 years.