Introduction
In December 2019, a cluster of severe, life-threatening cases of pneumonia was detected in China, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). On 11 March 2020, the World Health Organization (WHO) announced the coronavirus disease 2019 (COVID-19) pandemic.
Since the beginning of the pandemic, children have been reported all over the world to present with an asymptomatic or mild course of the disease [1–3].
However, it has been shown that chronic background illnesses, including heart disease, chronic lung disease, and/or asthma, developmental delay, diabetes, immune compromise, malignancy, and obesity, can trigger severe COVID-19, including in children [4]. SARS-CoV-2-related death in children is rare. In the United States, as of February 2023, there were > 16 million cases of COVID-19 and 2098 COVID-19-associated deaths in children < 18 years of age (< 0.01%) reported to the Center for Disease Control and Prevention (CDC) [5].
This work aimed to analyze the COVID-19 course in severely ill pediatric patients, hospitalized between March 2020 and September 2022. The analysis was conducted especially in terms of clinical presentation, laboratory findings, and outcomes. The study was designed to identify possibilities for the prediction of the severity of COVID-19 in hospitalized children.
Material and methods
Because of the COVID-19 pandemic announced by the WHO and the increasing incidence in Poland, the Department of Infectious Diseases and Paediatrics was ordered to treat children with COVID-19. The University Children’s Hospital was selected to provide highly specialized diagnosis and treatment, if necessary. These organizational solutions centralized the care for children with COVID-19 in the southern region of Poland.
On 23 March 2020, the first children with COVID-19 were admitted to the hospital, and the prospective study was started. Up to 30 September 2022, 2338 children were hospitalized.
Data were collected in all successively admitted patients, based on a standard protocol. On admission, after taking the medical history and performing a physical examination, a planned set of laboratory tests was taken in all patients, including complete blood count (CBC) parameters, C-reactive protein (CRP), alanine transaminase (ALT), lactate dehydrogenase (LDH), creatine kinase (CK), ferritin, and procalcitonin (PCT). All children with a severe course of COVID-19 had imaging tests taken (i.e., lung ultrasound (LU), chest X-ray, and high-resolution computed tomography (HRCT)). All children were subject to careful medical observation and treatment in accordance with current guidelines. Data on the course of hospitalization were also recorded in a planned and strictly structured manner in a specially designed database (length of hospitalization, length of oxygen treatment, antiviral treatment, systemic steroid therapy, pediatric intensive care unit (PICU) admission, respiratory therapy, and deaths).
Definitions
Owing to the absence of a consensus definition of the disease’s severity in children, different definitions have been used in studies categorizing its level [6]. In this study, we used severity classification for acute SARS-CoV-2 infection provided in the multicenter interim guidance on the use of antivirals for children with COVID-19 [4, 7].
The severe case was diagnosed in a patient with pneumonia, age 0–18 years, laboratory-confirmed COVID-19, and a new requirement for supplemental oxygen or increased requirement from baseline without new or increased need for ventilatory support (noninvasive or invasive). Patients diagnosed with multisystem inflammatory syndrome in children (MIS-C) were excluded.
According to the WHO and the National Institute of Public Health recommendations [8, 9], COVID-19 was diagnosed using a positive reverse transcription and real-time polymerase chain reaction (RT-PCR) and, since 30 October 2020, using the second-generation antigen tests based on a nasopharyngeal swab performed in certified laboratories.
Radiographic and ultrasonographic (USG) pneumonia diagnoses were based on the interpretation by the treating physician. Lower respiratory infections were diagnosed based on clinical presentation, LU, chest X-rays, and HRCT.
Remdesivir was used in our departments according to the Food and Drug Administration’s (FDA) and European Medicines Agency’s (EMA’s) recommendations [10, 11].
Statistical analysis
In the first stage of the analysis, the population of children with severe COVID-19 was described in detail. Next, among children diagnosed with severe COVID-19, the following subgroups of patients were identified for further analysis: 1) children with severe COVID-19, who did not require mechanical ventilation and 2) children requiring mechanical ventilation. In addition, while evaluating the collected data, it was decided to select a third subgroup of patients for analysis – children who died due to severe COVID-19. In the final stage of the analysis, the data of children with severe COVID-19 were also compared with the data of children hospitalized due to COVID-19, but who did not meet the criteria for severe COVID-19. To reduce the risk of error associated with the lack of complete data, data from only one center were included in the analysis in this part of the study. The data for comparison came from the register of COVID-19 patients previously published by the authors of the study and included 1407 patients hospitalized between March 2020 and April 2022.
The statistical analysis was performed using SPSS ver. 27 software (Armonk, NY, USA). Results were presented based on the parameters of descriptive statistics, including the mean values and standard deviations (SD) for the quantitative variables with a normal distribution or median values with the interquartile range for non-normally distributed data. Categorical variables were presented as numbers with percentages. Qualitative values were compared by the χ2 test. The Kruskal-Wallis test was used for the analysis of the continuous variables investigated in the study. In all cases of statistical significance, a pairwise comparison between the groups was performed using a post-hoc test. In all analyses, a p-value < 0.05 was considered statistically significant.
Results
Characteristics of children with severe COVID-19
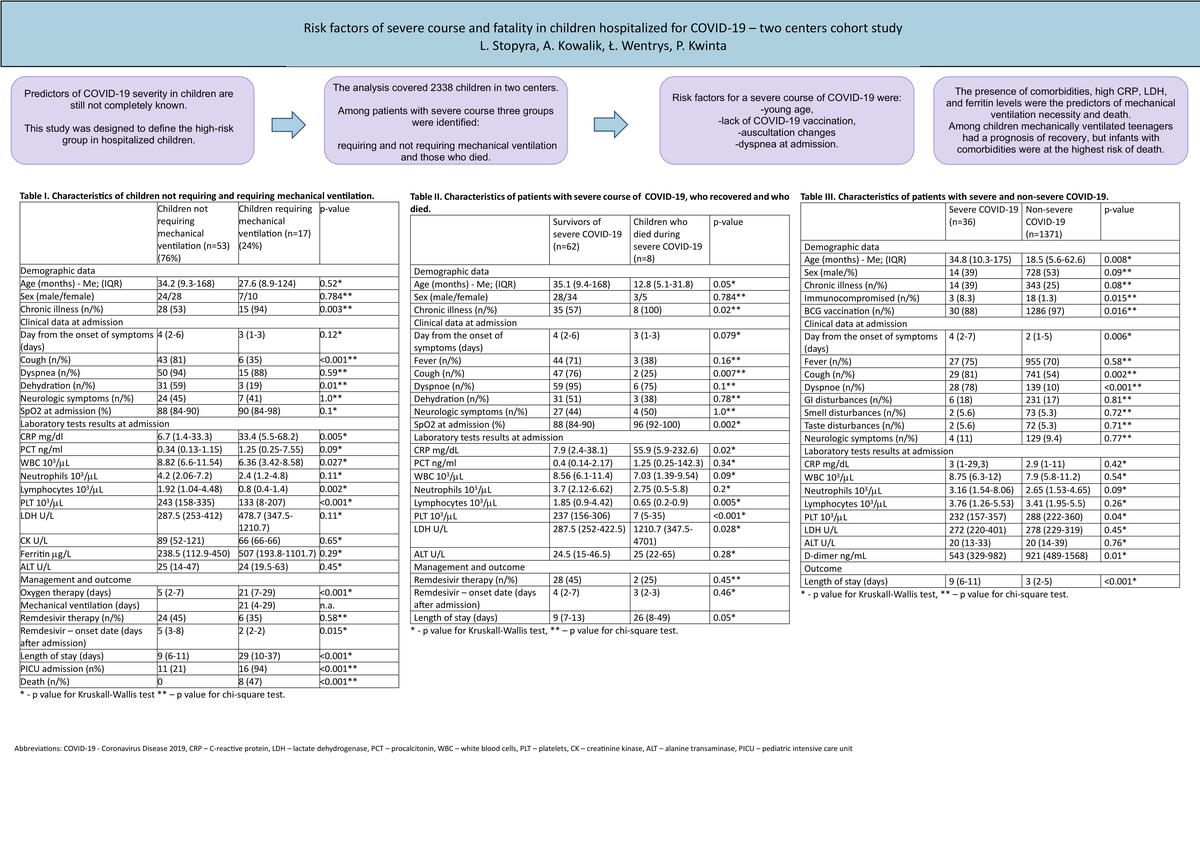
Seventy among 2338 children hospitalized in two centers (3%) met the criteria of COVID-19 severe course. The median age was 32.65 with a slight female prevalence (54%). In 43 (61%), comorbidities were present. Most often, neurological (26%), cardiological (14%), and genetic diseases (11%), and obesity occurred. Nine (13%) patients had multiple underlying conditions. On admission, medium SpO2 was 89%; 65 (93%) patients presented with dyspnea, 49 (70%) with cough, 31 (44%) with neurological symptoms, and 34 (49%) with dehydration. The medium length of stay was 13.9 days. Twenty-seven (37%) patients required admission to the PICU and 8 (11%) died. Although the vaccination against COVID-19 was available in Poland from 7 June 2021 for 12–15-year-olds and from 4 December 2021 for 5–11-year-olds, nobody in our cohort was vaccinated.
Risk factors for the need for mechanical ventilation in children with severe COVID-19
Among 70 children with severe COVID-19, 53 (76%) required passive oxygen therapy and 17 (24%) mechanical ventilation. A comparison of selected demographic and clinical data in the groups of children not requiring and requiring ventilator therapy is presented in Table I.
Table I
Characteristics of children not requiring and requiring mechanical ventilation
| Parameter | Children not requiring mechanical ventilation (n = 53) (76%) | Children requiring mechanical ventilation (n = 17) (24%) | P-value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age [months] Me (IQR) | 34.2 (9.3–168) | 27.6 (8.9–124) | 0.52* | |
| Sex (male/female) | 24/28 | 7/10 | 0.784** | |
| Chronic illness, n (%) | 28 (53) | 15 (94) | 0.003** | |
| Clinical data at admission | ||||
| Day from the onset of symptoms [days] | 4 (2–6) | 3 (1–3) | 0.12* | |
| Cough, n (%) | 43 (81) | 6 (35) | < 0.001** | |
| Dyspnea, n (%) | 50 (94) | 15 (88) | 0.59** | |
| Dehydration, n (%) | 31 (59) | 3 (19) | 0.01** | |
| Neurologic symptoms, n (%) | 24 (45) | 7 (41) | 1.0** | |
| SpO2 at admission (%) | 88 (84–90) | 90 (84–98) | 0.1* | |
| Laboratory test results at admission | ||||
| CRP [mg/dl] | 6.7 (1.4–33.3) | 33.4 (5.5–68.2) | 0.005* | |
| PCT [ng/ml] | 0.34 (0.13–1.15) | 1.25 (0.25–7.55) | 0.09* | |
| WBC [103/µl] | 8.82 (6.6–11.54) | 6.36 (3.42–8.58) | 0.027* | |
| Neutrophils [103/µl] | 4.2 (2.06–7.2) | 2.4 (1.2–4.8) | 0.11* | |
| Lymphocytes [103/µl] | 1.92 (1.04–4.48) | 0.8 (0.4–1.4) | 0.002* | |
| PLT [103/µl] | 243 (158–335) | 133 (8–207) | < 0.001* | |
| LDH [U/l] | 287.5 (253–412) | 478.7 (347.5–1210.7) | 0.11* | |
| CK [U/l] | 89 (52–121) | 66 (66–66) | 0.65* | |
| Ferritin [µg/l] | 238.5 (112.9–450) | 507 (193.8–1101.7) | 0.29* | |
| ALT [U/l] | 25 (14–47) | 24 (19.5–63) | 0.45* | |
| Management and outcome | ||||
| Oxygen therapy [days] | 5 (2–7) | 21 (7–29) | < 0.001* | |
| Mechanical ventilation [days] | 21 (4–29) | n.a. | ||
| Remdesivir therapy, n (%) | 24 (45) | 6 (35) | 0.58** | |
| Remdesivir – onset date [days after admission] | 5 (3–8) | 2 (2–2) | 0.015* | |
| Length of stay [days] | 9 (6–11) | 29 (10–37) | < 0.001* | |
| PICU admission, n (%) | 11 (21) | 16 (94) | < 0.001** | |
| Death, n (%) | 0 | 8 (47) | < 0.001** | |
Children who required mechanical ventilation during COVID-19 were characterized by a higher incidence of chronic diseases (94% vs. 53%; p = 0.003). These children reported slightly earlier after the onset of the first symptoms (3 vs. 4 days). On admission, they manifested less frequent cough (35% vs. 81%; p < 0.001) and dehydration (19% vs. 59%; p = 0.01). The markers of inflammation were higher (median CRP 33.4 vs. 6.7 mg/dl; p = 0.005), and lymphopenia and thrombocytopenia were observed. Moreover, higher concentrations of ferritin and LDH were found. It can be seen that 45% and 35% of children in both groups respectively have been treated with remdesivir; the difference is not statistically significant (p = 0.58). Medium length of stay was longer in the group of mechanically ventilated patients (29 vs. 9 days), as was the length of oxygen therapy (21 vs. 5 days). Eleven (21%) children with noninvasive oxygen support and 16 (94%) in the group with invasive mechanical ventilation were treated in a PICU (p < 0.001). Eight children requiring mechanical ventilation died during hospitalization and 9 recovered. The most significant difference between children who recovered, and those who died was age – 124 vs. 12.8 months.
Risk factors for death in children with severe COVID-19
Table II presents a comparison of data in groups of children with severe COVID-19 who survived and those who died during severe COVID-19. The children who died because of COVID-19 were of special interest to us. They all presented underlying chronic diseases (100% vs. 57%) and reported slightly earlier after the onset of the first symptoms (3 vs. 4 days). On admission, they presented less frequently with cough (25% vs. 76%; p = 0.007) and normal SatO2 (96% vs. 88%; p = 0.002). It is worth noting that the markers of inflammation were higher (median CRP 55.9 vs. 7.9 mg/dl; p = 0.02), and deep lymphopenia (0.65 vs. 1.85 × 103/µl, p = 0.005), and thrombocytopenia (7 vs. 237 × 103/µl, p < 0.001) were observed. Significant differences in ferritin (252.1 vs. 1521.5 μg/l, p < 001) and LDH (1210.7 vs. 287.5 U/l, p = 0.028) levels were found. The time of hospitalization was much shorter in the group of survivors (9 vs. 26 days). All eight fatalities were the result of COVID-19. All children had underlying diseases but they were in a stable condition when they contracted COVID-19. The clinical manifestation of the disease leading to death was also typical for COVID-19, not for underlying diseases.
Table II
Characteristics of patients with severe course of COVID-19, who recovered and who died
| Parameter | Survivors of severe COVID-19 (n = 62) | Children who died during severe COVID-19 (n = 8) | P-value |
|---|---|---|---|
| Demographic data | |||
| Age [months] Me (IQR) | 35.1 (9.4–168) | 12.8 (5.1–31.8) | 0.05* |
| Sex (male/female) | 28/34 | 3/5 | 0.784** |
| Chronic illness, n (%) | 35 (57) | 8 (100) | 0.02** |
| Clinical data at admission | |||
| Day from the onset of symptoms [days] | 4 (2–6) | 3 (1–3) | 0.079* |
| Fever, n (%) | 44 (71) | 3 (38) | 0.16** |
| Cough, n (%) | 47 (76) | 2 (25) | 0.007** |
| Dyspnea, n (%) | 59 (95) | 6 (75) | 0.1** |
| Dehydration, n (%) | 31 (51) | 3 (38) | 0.78** |
| Neurologic symptoms, n (%) | 27 (44) | 4 (50) | 1.0** |
| SpO2 at admission (%) | 88 (84–90) | 96 (92–100) | 0.002* |
| Laboratory test results at admission | |||
| CRP [mg/dl] | 7.9 (2.4–38.1) | 55.9 (5.9–232.6) | 0.02* |
| PCT [ng/ml] | 0.4 (0.14–2.17) | 1.25 (0.25–142.3) | 0.34* |
| WBC [103/µl] | 8.56 (6.1–11.4) | 7.03 (1.39–9.54) | 0.09* |
| Neutrophils [103/µl] | 3.7 (2.12–6.62) | 2.75 (0.5–5.8) | 0.2* |
| Lymphocytes [103/µl] | 1.85 (0.9–4.42) | 0.65 (0.2–0.9) | 0.005* |
| PLT [103/µl] | 237 (156–306) | 7 (5–35) | < 0.001* |
| LDH [U/l] | 287.5 (252–422.5) | 1210.7 (347.5–4701) | 0.028* |
| ALT [U/l] | 24.5 (15–46.5) | 25 (22–65) | 0.28* |
| Management and outcome | |||
| Remdesivir therapy, n (%) | 28 (45) | 2 (25) | 0.45** |
| Remdesivir – onset date [days after admission] | 4 (2–7) | 3 (2–3) | 0.46* |
| Length of stay [days] | 9 (7–13) | 26 (8–49) | 0.05* |
Risk factors for severe course of COVID-19 in children
A comparison of selected demographic, clinical, and laboratory factors is presented in Table III.
Table III
Characteristics of patients with severe and non-severe COVID-19
| Parameter | Severe COVID-19 (n = 36) | Non-severe COVID-19 (n = 1371) | P-value |
|---|---|---|---|
| Demographic data | |||
| Age [months] Me (IQR) | 34.8 (10.3–175) | 18.5 (5.6–62.6) | 0.008* |
| Sex (male/%) | 14 (39) | 728 (53) | 0.09** |
| Chronic illness, n (%) | 14 (39) | 343 (25) | 0.08** |
| Immunocompromised, n (%) | 3 (8.3) | 18 (1.3) | 0.015** |
| BCG vaccination, n (%) | 30 (88) | 1286 (97) | 0.016** |
| Clinical data at admission | |||
| Day from the onset of symptoms (days) | 4 (2–7) | 2 (1–5) | 0.006* |
| Fever, n (%) | 27 (75) | 955 (70) | 0.58** |
| Cough, n (%) | 29 (81) | 741 (54) | 0.002** |
| Dyspnea, n (%) | 28 (78) | 139 (10) | < 0.001** |
| GI disturbances, n (%) | 6 (18) | 231 (17) | 0.81** |
| Smell disturbances, n (%) | 2 (5.6) | 73 (5.3) | 0.72** |
| Taste disturbances, n (%) | 2 (5.6) | 72 (5.3) | 0.71** |
| Neurologic symptoms, n (%) | 4 (11) | 129 (9.4) | 0.77** |
| Laboratory test results at admission | |||
| CRP [mg/dl] | 3 (1–29,3) | 2.9 (1–11) | 0.42* |
| WBC [103/µl] | 8.75 (6.3–12) | 7.9 (5.8–11.2) | 0.54* |
| Neutrophils [103/µl] | 3.16 (1.54–8.06) | 2.65 (1.53–4.65) | 0.09* |
| Lymphocytes [103/µl] | 3.76 (1.26–5.53) | 3.41 (1.95–5.5) | 0.26* |
| PLT [103/µl] | 232 (157–357) | 288 (222–360) | 0.04* |
| LDH [U/l] | 272 (220–401) | 278 (229–319) | 0.45* |
| ALT [U/l] | 20 (13–33) | 20 (14–39) | 0.76* |
| D-dimer [ng/ml]L | 543 (329–982) | 921 (489–1568) | 0.01* |
| Outcome | |||
| Length of stay [days] | 9 (6–11) | 3 (2–5) | < 0.001* |
The patients with severe COVID-19 were older (median age: 34.8 vs. 18.5 months, p = 0.008). On admission, they more often presented cough (81% vs. 54%, p < 0.001), dyspnea (78% vs. 10%, p < 0.001), and auscultation changes (77.8% vs. 18.8%, p < 0.001).
Interestingly, underlying chronic diseases were only slightly more frequent in the group of severely ill children (39% vs. 25%, p = 0.08). Only immunosuppression was much more common in the group with the severe course (8.3% vs. 1.3%, p < 0.001). Laboratory test results were similar in both groups; only small differences in platelet count and D-dimers on admission were found.
There was a significant difference between the studied groups in BCG vaccination (97% among the patients with mild course and only 88% in the group with severe course, p = 0.002).
Discussion
During the 3 years of the COVID-19 pandemic, the course of the disease has been changing. It is due to the succession of SARS-CoV-2 variants and growth of herd immunity. Since the beginning of the pandemic, children have been considered to have a good prognosis. Now the number of cases is small and the course of COVID-19 is usually mild, but hospitalization among children has increased since the emergence of contagious SARS-CoV-2 variants and the achievement of a high vaccination rate in adults. The prediction of severity is still important to help in medical decision-making.
As a first step, the definition of severity should be described. There is no consensus definition of severe pediatric COVID-19, and in previous studies, different definitions were used. The most popular is the WHO definition, in which a severe course can be diagnosed in a child with clinical signs of pneumonia (cough or difficulty in breathing + fast breathing or chest wall indrawing + at least one of the following: SpO2 < 90%; very severe chest wall indrawing, grunting, central cyanosis, or presence of any other general danger sign (inability to breastfeed or drink, lethargy or unconsciousness, or convulsions)) [12]. While the diagnosis can be made on clinical grounds, chest imaging (radiograph, computed tomography (CT) scan, ultrasound) may assist in diagnosis and identify or exclude pulmonary complications [13].
Other investigators have expressed their own definitions. Zachariah et al. [14] defined severity as the need for ventilatory or hemodynamic support, and Dong et al. [15] defined it as having respiratory symptoms, dyspnea, and hypoxemia. Armin et al. [16] included a set of events that indicate the severity of COVID-19: using a ventilator, oxygen with reservoir, taking a vasoactive drug, intravenous immunoglobulin (IVIG) or corticosteroid therapy, and death.
In this study, we used severity classification for acute SARS-CoV-2 infection provided in the multicenter interim guidance on the use of antivirals for children with COVID-19 [6, 7]. This was because they met the requirements for the use of antivirals in children and allowed for MIS-C exclusion.
The characteristics of our patients with the severe course were similar to those in other studies, but a difference concerned the frequency of comorbidities, which were only slightly more common in patients with the severe course. Other authors have described the overwhelming prevalence of patients with comorbidities [17–19]. It might have been due to the different definitions of disease severity in other studies.
Nevertheless, in our cohort in the group of patients requiring invasive oxygen treatment, the prevalence of those with underlying conditions was significant, and 100% of children who died because of COVID-19 had comorbidities. The most frequent were the same as in other studies – genetic conditions, neurologic conditions, metabolic conditions, cardiovascular disease, obesity, diabetes mellitus, chronic pulmonary diseases, and immunosuppression [20, 21].
All children who died presented underlying diseases: three had cardiological conditions (congenital heart defects, cardiomyopathy), two were premature, there was 1 case of cri du chat syndrome, one of polycystic kidney disease, and one of acute lymphoblastic leukemia (ALL). Among all children with the severe course, there was a significant predominance of those with neurological disorders, development retardation, cardiovascular diseases, genetic disorders, and neonates. Thirteen percent of children had multiple comorbidities. It was also found by other authors that having multiple underlying conditions is associated with an increased risk of severe disease [19, 22]. In multicenter studies of children admitted to PICUs with COVID-19 and COVID-19-related deaths, most of the patients had one or more underlying conditions [20, 23, 24].
Although immune compromise has been reported as an underlying condition in children with severe COVID-19, the relationship between immune compromise and severe COVID-19 has not been well established. In small surveys of children who developed COVID-19 while receiving immunosuppressive medications, COVID-19 was mild [25, 26]. In another study that included 8 children with rheumatic disease, active disease and use of glucocorticoids were associated with severe disease [5, 27]. In our study, immunosuppression was significantly more common in the group of children with a severe course of the disease.
Childhood cancer appears to be associated with increased severity of COVID-19. In a global registry study of COVID-19 in children with cancer including 1500 patients, severe or critical infection and mortality were higher than in the general pediatric population [5, 28]. Among children with cancer, the severe disease has been associated with intensive chemotherapy, neutropenia, lymphopenia, comorbidity, and coinfection [28, 29]. There was only one child with ALL among children with severe COVID-19 in our cohort. Children with hematologic malignancy do not appear to be at greater risk of severe COVID-19 than children with nonhematologic malignancy [5, 30].
Other conditions that may be associated with severe disease in children, but for which the evidence is inconsistent, include age < 1 year [19, 24, 31, 32], Down syndrome [33, 34] and prematurity [35, 36]. Our study confirmed them as risk factors for severe pediatric COVID-19.
In our cohort, children who required mechanical ventilation manifested cough and dehydration more often, contrary to cases described in other studies. Also, similar laboratory results of higher markers of inflammation, lymphopenia, and thrombocytopenia with higher concentrations of ferritin and LDH have been reported by other authors [16, 18]. In children who died because of COVID-19, inflammatory markers, leucopenia, thrombocytopenia, LDH, and ferritin level were extremely high (Table II).
In this study, the BCG vaccination was also considered to be a factor that influenced COVID-19 severity, because it was hypothesized that countries without widespread tuberculosis prevention policies had a higher percentage of severe cases (Italy, France, and Spain) than countries that adopted long-term widespread prevention (Japan, Denmark, and Korea). In Poland, antituberculosis BCG vaccination was obligatory, so in our pediatric study groups, over 95% of patients had been vaccinated. A lack of BCG vaccination was found in 12% of children with severe COVID-19 and 3% in other hospitalized children. Various publications have described the results of the first association between BCG vaccination and COVID-19 case severity, but these have concerned only adults [37].
Our study had several limitations. The recommendations regarding the rules for COVID-19 testing, indications for hospitalization, and treatment availability were changing during the pandemic. This could have influenced the number of hospitalized and treated children, as well as the length of hospitalization.
Almost all children taking part in our study were Caucasian. In Poland, ethnic diversity is low, with racial minorities constituting a very small proportion of the population. In other reports, Black African and Hispanic populations were significantly overrepresented among severe COVID-19 pediatric cases.
The next limitation is the relatively small number of patients with a severe course of COVID-19 in our study. Moreover, it cannot be ruled out that a child with severe disease could have been hospitalized and even could have died in another hospital in our region. Also, the numerous definitions of COVID-19 severity makes comparison with other studies difficult.
In conclusion, in our study, risk factors for a severe course of COVID-19 were: young age, lack of COVID-19 and BCG vaccination, auscultation changes, and dyspnea on admission. The presence of comorbidities, high CRP, LDH, and ferritin levels were the predictors of need for mechanical ventilation and death. Among children mechanically ventilated, teenagers generally had a favorable prognosis of recovery, whereas infants with comorbidities were at the highest risk of death. Our observations may be useful for defining the high-risk group for severe COVID-19 and could help to guide hospital admission and prevention of COVID-19 in pediatric patients.