Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
IMMUNOLOGY / CLINICAL RESEARCH
Identification and validation of immune pathway subtyping in glioblastoma to predict clinical prognosis and immunotherapy response in patients
1
Department of Neurosurgery, The First Affiliated Hospital of Jiamusi University, China
2
Department of Stomatology, The First Affiliated Hospital of Jiamusi University, China
Submission date: 2022-11-02
Final revision date: 2023-01-05
Acceptance date: 2023-01-16
Online publication date: 2023-02-04
Corresponding author
Yuyi Hou
Department of Stomatology, The First Affiliated Hospital of Jiamusi University, 154002, Jiamusi, China
Department of Stomatology, The First Affiliated Hospital of Jiamusi University, 154002, Jiamusi, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Glioblastoma (GBM) as a frequently diagnosed primary intracranial tumor has a significantly poor prognosis. Only a few studies have probed into the immune profile associated with GBM. This study explored the role of immune features of GBM in prognosis and immunotherapy response.
Material and methods:
GBM samples were subtyped by evaluating 15 immune-related pathways and genes using consensus clustering. GISTIC2 analyzed copy number variations and the impute package was used to perform methylation analysis. Immune characteristics were unveiled by using ssGSEA, ESTIMATE, and CIBERSORT. Immunotherapy and chemotherapeutic drug responses were calculated with the TIDE and pRRophetic package respectively. Weighted gene co-expression network analysis (WGCNA), Cox regression, Lasso, and stepAIC were used to develop a prognostic immune score (IMscore) model.
Results:
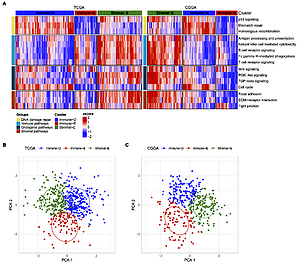
GBM was categorized into 3 subtypes: immune-deprived (Immune-D) (low enrichment of immune pathways and high enrichment of DNA damage repair pathways); stromal-enriched (Stromal-E) (high enrichment of immune pathways, oncogenic pathways and stromal pathways); and immune-enriched (Immune-E) (low enrichment of DNA damage repair pathways and high enrichment of immune pathways). Methylation differences were found in TWIST1, CDH2 and CDH1 among 3 subtypes. Immune-E responded better to immunotherapy, while Immune-D was more sensitive to chemotherapeutic drugs. This study established a prognostic model with five genes (OSMR, SPP1, CUL1, CTBP2, NGFR) for GBM.
Conclusions:
Three subtypes had different prognosis and response to immunotherapy and chemotherapy. A five-gene prognostic model was robust to predict prognosis in GBM as well as pan-cancer. The subtyping and prognostic model may facilitate individualized prognosis management and personalized therapeutic intervention.
Glioblastoma (GBM) as a frequently diagnosed primary intracranial tumor has a significantly poor prognosis. Only a few studies have probed into the immune profile associated with GBM. This study explored the role of immune features of GBM in prognosis and immunotherapy response.
Material and methods:
GBM samples were subtyped by evaluating 15 immune-related pathways and genes using consensus clustering. GISTIC2 analyzed copy number variations and the impute package was used to perform methylation analysis. Immune characteristics were unveiled by using ssGSEA, ESTIMATE, and CIBERSORT. Immunotherapy and chemotherapeutic drug responses were calculated with the TIDE and pRRophetic package respectively. Weighted gene co-expression network analysis (WGCNA), Cox regression, Lasso, and stepAIC were used to develop a prognostic immune score (IMscore) model.
Results:
GBM was categorized into 3 subtypes: immune-deprived (Immune-D) (low enrichment of immune pathways and high enrichment of DNA damage repair pathways); stromal-enriched (Stromal-E) (high enrichment of immune pathways, oncogenic pathways and stromal pathways); and immune-enriched (Immune-E) (low enrichment of DNA damage repair pathways and high enrichment of immune pathways). Methylation differences were found in TWIST1, CDH2 and CDH1 among 3 subtypes. Immune-E responded better to immunotherapy, while Immune-D was more sensitive to chemotherapeutic drugs. This study established a prognostic model with five genes (OSMR, SPP1, CUL1, CTBP2, NGFR) for GBM.
Conclusions:
Three subtypes had different prognosis and response to immunotherapy and chemotherapy. A five-gene prognostic model was robust to predict prognosis in GBM as well as pan-cancer. The subtyping and prognostic model may facilitate individualized prognosis management and personalized therapeutic intervention.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.