Obesity is an increasing morbid condition with epidemic features associated with cardiovascular risk factors [1]. Currently, bariatric surgery for severely obese patients has proven to be the most effective treatment option, with the number of procedures performed increasing on a global scale [2]. The Swedish Obese Subjects (SOS) study [3] discovered that patients with obesity who underwent bariatric surgery had a 53% lower risk of mortality from cardiovascular disease when compared to patients who did not have the procedure. One mechanism by which this reduction may occur is through alteration of lipid profiles of patients. Dyslipidemia is improved following bariatric surgery and the extent of improvement varies depending on the surgical approach utilized [4].
PCSK9 is a key regulator of low-density lipoprotein-cholesterol (LDL-C) via enhancing LDL receptor internalization and endosomal degradation. PCSK9 plasma levels have been demonstrated to predict cardiovascular (CVD) risk [5–8]. Interestingly, some studies have found a correlation between PCSK9 concentrations and fat mass [9]. Although caloric restriction lowers PCSK9 concentration in adults [10], bariatric surgery has recently been found to lower plasma LDL-C as well as PCSK9 levels simultaneously [11]. However, the magnitude of such a reduction is not known and there has been no meta-analysis evaluating the effects of bariatric procedures on plasma PCSK9 concentrations. This meta-analysis focuses on PCSK9 changes in obese patients following bariatric surgery.
Methods. PubMed, Scopus, Embase, and Web of Science were searched from inception to June 1st, 2021 using the following keywords in titles and abstracts (also when used with MESH terms): (“bariatric surgery” OR gastroplast* OR “gastric bypass” OR “Roux-en-Y” OR “gastric band” OR “biliopancreatic diversion” OR gastrectom* OR “duodenal switch” OR “weight loss surgery” OR “gastrointestinal diversion” OR gastroenterostom* OR “jejunoileal bypass” OR “obesity surgery” OR “weight-loss surgery” OR “bariatric procedure” OR “sleeve surgery” OR “metabolic surgery”) AND (PCSK9 OR PCSK-9 OR “proprotein convertase subtilisin kexin type 9” OR “proprotein convertase subtilisin/kexin type 9” OR NARC-1 OR “neuronal apoptosis regulated convertase-1”). Only peer-reviewed original papers written in English were considered for inclusion. All forms of bariatric surgery were considered in the study. Papers must have documented PCSK9 before and after surgery to be considered for inclusion.
Quality assessment. The Newcastle-Ottawa Scale (NOS) was utilized to estimate the quality of the studies included in this meta-analysis [12, 13]. Three aspects of each qualified study are considered for this scale: (1) the selection of the patients in the studies (4 items), (2) the comparability of the studied populations (one item) and (3) the outcome of interest (3 items).
Quantitative data synthesis. The meta-analysis was conducted by Comprehensive Meta-Analysis (CMA) V2 software (Biostat, NJ) [14]. The weighted mean differences (WMDs) with relevant CIs were determined for continuous outcomes. From each group sample sizes, means, and standard deviations were obtained for each relevant outcome to calculate WMDs. Overall estimate of effect size was measured by a random effects meta-analysis. To account for the heterogeneity of studies with regard to study design, characteristics of the populations under investigation and treatment duration, a random-effects model (using the DerSimonian-Laird method) and the generic inverse variance weighting approach were utilized [15]. A sensitivity analysis employing the leave-one-out approach (i.e., deleting one study each time and repeating the analysis) was applied to analyze the effect of each study on the overall effect size.
Results. Among 90 published studies identified in the systematic database search, 34 were directly related to the topic of this study. Among them, 30 studies were excluded after careful evaluation (20 studies were reviews, 7 studies did not match the inclusion criteria and 3 studies did not report sufficient data). Therefore, 4 studies which evaluated PCSK9 after bariatric surgery were included (Table I) [16]. Figure 1 depicts the process of study selection.
Table I
Characteristics of studies measuring PCSK9
| No. of patients | Patients | Clinical result | Control | Treatment | Follow-up | Study design | Study, year | |
|---|---|---|---|---|---|---|---|---|
| P-value | PCSK9 | |||||||
| 20 | Morbidly obese subjects | < 0.003 | Significant decrease in PCSK9 values | Nonobese healthy controls | Bariatric surgery | 6 months | Non-randomized interventional study | Zenti et al., 2020 [16] |
| 156 | Morbidly obese subjects | < 0.0001 | Significant change in PCSK9 values | – | SG/RYGB | 6 months | Prospective study | Blanchard et al., 2020 [9] |
| 15 | Morbidly obese subjects | Not mentioned | Significant change in PCSK9 values | – | RYGB | 6 months | Prospective study | Ghanim et al., 2018 [5] |
| 69 | Morbidly obese subjects | < 0.005 | Significant decrease in PCSK9 values | Severely obese patients | BPD-DS | 24 h 5 days 6 months 12 months | Randomized interventional study | Boyer et al., 2015 [11] |
Quality assessment of included studies. All the selected studies showed representativeness of the exposed cohorts, but most of them were distinguished by a lack of selection of the non-exposed cohort. Given that most of the studies did not include a non-exposed group, they were not assessed for comparability and adequacy of follow-up of cohorts. Finally, all included studies met the assessment of outcome. Quality assessment of the selected studies is presented in Table II.
Table II
Quality assessment of included studies
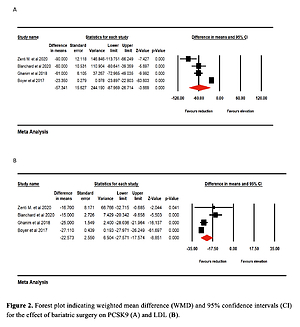
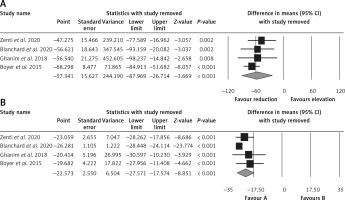
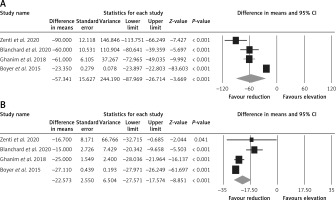
Effect of bariatric surgery on PCSK9 and LDL-C. The random-effects meta-analysis of 4 studies including 260 subjects demonstrated a significant decrease of plasma PCSK9 levels after bariatric surgery (WMD = –57.341 ng/ml, 95% CI: –87.969, –26.714, p < 0.001; I2 = 96.25%) (Figure 2 A). The reduction in PCSK9 was robust in the leave-one-out sensitivity analysis. Consistently, a significant decrease of LDL-C after bariatric surgery (WMD = –22.573 mg/dl, 95% CI: –27.571, –17.574, p < 0.001; I2 = 86.35%) was observed (Figure 2 B). The reductions in PCSK9 and LDL-C were robust in the leave-one-out sensitivity analysis (Figures 3 A, B).
Figure 2
Forest plot indicating weighted mean difference (WMD) and 95% confidence interval (CI) for the effect of bariatric surgery on PCSK9 (A) and LDL (B)
Meta-regression. Random-effects meta-regression was used to analyze the association between baseline BMI and the PCSK9-reducing effect of bariatric surgery. The results suggested a significant association between the changes in PCSK9 and baseline BMI (slope: 8.232; 95% CI: 5.622, 10.842; p < 0.001). The results must be interpreted with caution owing to the limited number of included studies.
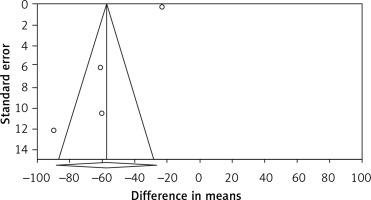
Publication bias. Evaluation for bias using Egger’s test (intercept = –5.206, standard error = 0.858; 95% CI = –8.899, –1.513, t = 6.066, df = 2, two-tailed p = 0.026) suggested publication bias, while Begg’s test (Kendall’s tau with continuity correction = –0.166, z = 0.339, two-tailed p-value = 0.734) suggested that there was no publication bias in the meta-analysis demonstrating bariatric surgery’s impact on PCSK9. Trim and fill correction identified one potentially “missing” study. In accordance with the “fail-safe N” test, 2961 missing papers would be required to lower the effect size to a non-significant (p < 0.001) level. The results must be interpreted with caution owing to the limited number of included studies (Figure 4).
Figure 4
Funnel plot detailing publication bias in the publications describing the effect of bariatric surgery on PCSK9
Discussion. This meta-analysis indicated a significant decrease of circulating PCSK9 levels after bariatric surgery. This is the first meta-analysis evaluating the impacts of bariatric surgery on PCSK9 as a regulator of plasma cholesterol levels. This finding is consistent with other reports suggesting a positive impact of bariatric surgery on cardiovascular risk factors [17–20]. The observed drop in circulating PCSK9 levels could be attributed to long-term weight loss caused by the bariatric procedure as well as sustained decreases in total food intake [11]. Total weight loss after bariatric surgery was found to be inversely associated with PCSK9 alterations [9]. It suggests that following bariatric surgery, PCSK9 levels were significantly decreased throughout chronic and significant weight loss. However, a recent study indicated that enhancing nutritional quality and physical activity did not result in reductions in circulating PCSK9 levels, implying that modest weight loss is not enough to reduce plasma PCSK9 concentration [21]. Furthermore, reduced PCSK9 levels in the chronic period may potentially be explained by better function and regulation of adipose tissue, as well as adipokine secretion following bariatric surgery. It was recently found that leptin and resistin may modulate PCSK9 and LDL-C levels [22]. Previous research also revealed that in response to bariatric surgery-induced weight loss and insulin sensitivity, adipose tissue plays an undetected role in PCSK9 homeostasis. Thus, it is feasible to speculate that physiological insulin levels may influence expression of hepatic PCSK9 and its circulating levels [16]. Alternatively, it is possible that the observed alterations in plasma PCSK9 levels are attributable to a change in metabolism of bile acids. Certainly, after Roux-en-Y gastric bypass (RYGB), bile acids in plasma have been shown to be significantly elevated in morbidly obese patients [23]. Increased bile acid concentrations can result in reduced PCSK9, as bile acids reduce hepatic mRNA and protein expression of PCSK9, an effect that is likely mediated by the activation of farnesoid X receptor [24].
Despite the novelty of this study, limitations include the small number of overall studies, restricting the generalizability of the findings. Moreover, in most of the studies there was no differentiation of free and total PCSK9. Finally, the included studies lacked control groups with other methods of weight loss to allow a direct comparison between different weight loss approaches with respect to their impact on plasma PCSK9 levels.
In conclusion, our findings imply that bariatric surgery is related to lower circulating levels of PCSK9. This finding may partly explain the improvement in lipid profile and cardiovascular risk that occurs following surgery. More research is needed to better understand the mechanisms underlying the influence of bariatric surgery on PCSK9 concentration, as well as to determine the clinical impact of the weight loss procedure on PCSK9 function [25–28].