Introduction
Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is a very rare musculoskeletal disease that encompasses a variety of osteoarticular disorders accompanied by dermatoses and was introduced by Chamot et al. [1] in 1987. The diagnostic criteria for SAPHO syndrome were developed by Kahn et al. [2] in 1994. SAPHO syndrome is classified along two different spectrums in adults: pustulo-hyperostotic psoriatic spondyloarthritis (PPHS) and chronic recurrent multifocal osteomyelitis (CRMO) [3].
There is some debate whether SAPHO syndrome is a form of spondyloarthritis (SpA) or psoriatic arthritis (PsA), or a disease in its own right [4, 5].
The first sign of arthritis is anterior chest wall involvement, most often the sternoclavicular joints and attachments of the first ribs to the sternum. The typical skin lesions are palmoplantar pustulosis (PPP), severe acne or acne inversa (hidradenitis suppurativa) [1–3, 6, 7].
In SAPHO syndrome, in addition to the clinical assessment, imaging studies are important in diagnosis. In early stages of the disease, bone scintigraphy is useful. On computed tomography (CT), erosions, sclerotic changes, and new bone formation are observed [8–10].
The cause of SAPHO is unknown, and no standard treatment protocols are available. Propionibacterium acne may play a role as a potential antigenic trigger [11–13]. The syndrome is often chronic and eventually self-healing.
Several pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-18 (IL-18), interleukin-23 (IL-23) and cytokines involved in angiogenesis such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and proinflammatory peptide endothelin-1 (ET-1) are considered to be involved in the inflammatory process in SpA [14–16].
A variety of studies have suggested that patients with PsA have an increased risk of metabolic syndrome (MetS), which is a clustering of cardiovascular risk factors, such us obesity, hypertension, dyslipidemia, and insulin resistance [17, 18]. There are no data concerning the risk of MetS in patients with SAPHO syndrome.
The aim of the study was to evaluate the impact of disease activity, selected serum cytokines, and therapy on MetS components in patients with SAPHO syndrome.
Material and methods
This study was approved by the local ethics committee of the Pomeranian Medical University in Szczecin. Informed consent was obtained from all patients.
We studied 46 SAPHO patients and included 30 healthy volunteers as controls. All patients were Caucasian. The diagnosis of SAPHO syndrome was made according to the Kahn criteria [2].
The following data were recorded: age, sex, disease duration, type of joint involvement, type of skin changes, bone scintigraphy results, comorbidities, cigarette smoking, and treatment.
Weight and height were measured to calculate the body mass index (BMI, kg/m2). The waist and hip circumference were measured to calculate the waist/hip ratio (WHR).
The patient’s pain due to the disease at the time of examination was assessed using a visual analogue scale (VAS).
We also assessed the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). This index has a possible score of 0–10, with a higher score indicating greater disease activity [19].
Blood was taken after at least 8 h of fasting for assessment of the erythrocyte sedimentation rate (ESR, mm/h, Westergren method), C-reactive protein (CRP, mg/dl) (turbidimetric nephelometry, rate reaction), total cholesterol (TC, mmol/l), high-density lipoprotein cholesterol (HDL-C, mmol/l), low-density lipoprotein cholesterol (LDL-C, mmol/l), and triglycerides (TG, mmol/l), measured according to standard procedures. HLA-B27 was determined using a BD Biosciences test (Becton, Dickinson and Company BD Biosciences, San Jose, CA, USA) based on flow cytometry and a BD FACSCanto II apparatus. Rheumatoid factor (RF) was measured using an ELISA kit.
In 34 SAPHO patients serum was stored at –80°C until analysis for IL-6, IL-18, IL-23, ET-1, VEGF, and EGF using a sensitive sandwich ELISA method: Human IL-6 Immunoassay Quantikine ELISA kit (the minimum detectable dose less than 0.7 pg/ml) (R&D Systems, Minneapolis, United States), Human IL-18 Quantitative ELISA kit (the minimum detectable dose less than 12.5 pg/ml) (MBL, Nagoya, Japan), Human IL-23 Immunoassay Quantikine ELISA kit (the minimum detectable dose less than 6.8 pg/ml), Human ET-1 Immunoassay Quantikine ELISA kit (the minimum detectable dose less than 0.087 pg/ml), Human VEGF Immunoassay Quantikine ELISA kit (the minimum detectable dose less than 5.0 pg/ml), and Human EGF Immunoassay Quantikine ELISA kit (the minimum detectable dose less than 0.7 pg/ml) (R&D Systems, Minneapolis, United States). All analyses and calibrations were performed in duplicate according to the manufacturer’s instructions, and read using a BioTek PowerWaveXS spectrophotometer (Winooski, VT, USA).
The NCEP-ACT III criteria were used to identify subjects with MetS [20].
Statistical analysis
Data distributions were assessed using the Kolmogorov-Smirnov test. Data are described as mean ± standard deviation and median (Q1, Q3). The R values of correlations were determined and p < 0.05 was considered significant. The groups were compared using Student’s t-test, the Mann-Whitney U test and the Kruskal-Wallis test. To assess parameters, Pearson’s chi-squared test (χ2), logistic regression analysis, and step-wise analysis were performed. The level of significance was set at p < 0.05. The statistical analysis was performed using Statistica version 8.0 (StatSoft, Inc., Tulsa, OK, USA).
Results
The clinical and laboratory characteristics of the study group and controls are presented in Table I.
Table I
Clinical and laboratory characteristics of the study group and controls
[i] Data are presented as number (%), mean ± standard deviation, median (Q1, Q3). BASDAI – Bath Ankylosing Spondylitis Disease Activity Index, CRP – C-reactive protein, EGF – epidermal growth factor, ESR – erythrocyte sedimentation rate, ET-1 – endothelin-1, F – female, IL-6 – interleukin-6, IL-18 – interleukin-18, IL-23 – interleukin-23; M – male, n – number of patients, SAPHO – synovitis acne pustulosis hyperostosis osteitis syndrome, VAS pain – visual analogue scale of patient’s pain, VEGF – vascular endothelial growth factor, WHR – waist/hip ratio.
The most prevalent comorbidity was hypertension, followed by hypothyroidism, diabetes and depression (Table II).
Table II
The comorbidities in synovitis acne pustulosis hyperostosis osteitis syndrome patients
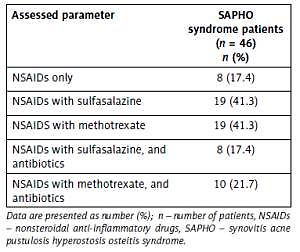
All patients were treated with non-steroidal anti-inflammatory drugs (NSAIDs), which were effective (on the basis of the reduction of clinical and laboratory activity of the disease) only in 17.4% of patients. In 41.3% of patients, sulfasalazine 1 g/day or methotrexate (MTX) 15 mg/day was added. In 39.1% of patients, with active skin changes, antibiotics were added (doxycycline 1× 100 mg/day (50%) or azithromycin 2 × 500 mg weekly (50%)) (Table III).
Table III
Treatment regimens used in synovitis acne pustulosis hyperostosis osteitis syndrome patients
No significant correlations were found between disease duration, VAS and BASDAI with serum levels of the selected cytokines (all p > 0.05).
Serum IL-6 levels were higher in SAPHO patients than in the control group (p = 0.09) (Table I). There was a positive correlation between IL-6 and CRP (r = 0.72; p = 0.00005), and ESR (r = 0.53; p = 0.005) and WHR (r = 0.82; p = 0.0002).
Serum IL-18 levels were higher in SAPHO patients than in controls (p = 0.01). Serum IL-23 levels were higher in SAPHO patients than in the control group (p = 0.01). No differences were found between SAPHO patients and controls in terms of ET-1, VEGF, and EGF levels (all p > 0.05) (Table I).
No correlations were found between serum IL-18, IL-23, VEGF, EGF, ET-1 and disease activity assessed by VAS, CRP, ESR, BASDAI in the SAPHO group (all p > 0.05) (data not shown).
SAPHO patients had higher BMI than controls (p = 0.02) (Table I). Metabolic syndrome criteria were present in 9 (19.5%) patients. SAPHO patients treated with MTX compared to those not treated with MTX had a higher BMI (28.4 ±3.9 vs. 26.1 ±3.3, p = 0.05) and higher prevalence of MetS (26.3% vs. 7.4%, p = 0.04). There were no differences between serum markers of the lipid profile between SAPHO and controls (all p > 0.05).
There was a positive correlation of TC with VAS (r = 0.55; p = 0.04) and with IL-23 (r = 0.63; p = 0.02) in SAPHO patients. SAPHO patients with VAS > 40 had higher TC levels compared to those with VAS ≤ 40 (235.3 ±30.6 vs. 177.0 ±37.2 mmol/l, p = 0.004). There was a negative correlation between TC and EGF in SAPHO patients (r = –0.74; p = 0.03).
There was a negative correlation of HDL-C with age (r = –0.41; p = 0.04), BASDAI (r = –0.51; p = 0.02), and with BMI (r = –0.65; p = 0.001) in SAPHO patients. SAPHO patients with BASDAI > 4 had lower HDL-C levels compared to those with BASDAI ≤ 4 (54.2 ±14.2 vs. 68.8 ±11.6 mmol/l, p = 0.02). SAPHO patients with BMI > 30.0 compared to those with BMI ≤ 30.0 had lower HDL-C levels (41.0 ±13.0 vs. 67.5 ±11.9 mmol/l, p = 0.009). There was a positive correlation between HDL-C and IL-23 (r = 0.67; p = 0.01).
There was a positive correlation of LDL-C with ESR (r = 0.45; p = 0.04), ET-1 (r = 0.47; p = 0.04), and with EGF (r = 0.66; p = 0.01) in SAPHO patients. SAPHO patients with VAS > 40 had higher LDL-C levels compared to those with VAS ≤ 40 (147.6 ±30.0 vs. 102.0 ±32.5 mmol/l, p = 0.01). SAPHO patients treated with MTX had higher LDL-C levels compared to those not treated with MTX (151.5 ±30.7 vs. 120.5 ±35.2 mmol/l, p = 0.04). SAPHO patients with BMI > 30.0 compared to those with BMI ≤ 30.0 kg/m2 had higher LDL-C levels (170.3 ±27.0 vs. 126.6 ±32.2 mmol/l, p = 0.02).
There was also a positive correlation of TG with age (r = 0.44; p = 0.03), BASDAI (r = 0.51; p = 0.02), and with BMI (r = 0.52; p = 0.01) in SAPHO patients. SAPHO patients with BASDAI > 4 had higher TG levels compared to those with BASDAI ≤ 4 (167.3 ±96.5 vs. 93.0 ±34.4 mmol/l, p = 0.01). SAPHO patients with BMI > 25.0 kg/m2 compared to those with BMI ≤ 25.0 kg/m2 had higher TG levels (166.7 ±89.2 vs. 98.2 ±24.6 mmol/l, p = 0.01). SAPHO patients treated with MTX had higher TG levels compared to those not treated with MTX (159.9 ±82.3 vs. 93.3 ±44.6 mmol/l, p = 0.01).
To assess parameters associated with serum IL-6, IL-18, IL-23, ET-1, VEGF, and EGF, Pearson’s chi-squared test (χ2), logistic regression analysis, and step-wise analysis were performed and no significant associations were found (all p > 0.05). There was no effect of age or sex on the results of the study.
There was no influence of treatment with NSAIDs, sulfasalazine or antibiotics on lipid profile markers in SAPHO patients.
Discussion
In our study, we investigated SAPHO syndrome, a rare musculoskeletal disease, and found that SAPHO was more common in middle-aged women, and that the most prevalent skin change was PPP. Other authors have confirmed the same associations [5, 21–23].
In our previously published study, we found that SAPHO patients with PPP compared with SpA patients without PPP had shorter disease durations, lower prevalence of HLA-B27 antigen, and lower BASDAI. Additionally, in SAPHO patients increased serum levels of IL-18 were associated with increased risk of PPP [24].
Serum IL-6 is a pro-inflammatory cytokine considered to be related to obesity development [25]. In our study serum IL-6 correlated positively with WHR, which is a marker of abdominal obesity.
We previously reported that serum IL-18 was higher in SAPHO patients with increased BMI [16]. Serum IL-18 is associated with atherosclerosis, which could suggest that SAPHO patients have increased risk of atherosclerosis stimulated by IL-18, and being overweight could be connected with increased secretion of IL-18 [16, 26].
A variety of studies have suggested that patients with PsA have an increased risk of MetS, which is a clustering of cardiovascular risk factors, such us obesity, hypertension, dyslipidemia, and insulin resistance [17, 18]. There are no data concerning the risk of MetS in patients with SAPHO syndrome. Our study was the first to present the specific components of the MetS in SAPHO patients. We found that disease activity correlated positively with levels of TC and LDL-C, which are well-known markers of the risk of cardiovascular disease. This suggests an increased risk of cardiovascular disease in this group of patients.
Interleukin-23 is considered to be involved in the pathogenesis of SpA [15, 27]. Interestingly, we found a positive correlation between serum IL-23 and HDL-C in our patients. This could suggest a protective effect of IL-23 on MetS risk in SAPHO patients. Lang et al. [28] explained that cholesterol metabolism interacts with the IL-23, IL-17 and granulocyte colony-stimulating factor (G-CSF) axis in hematopoietic stem cell mobilization and differentiation to inflammatory cells that participate in atherosclerosis in mice. The influence of IL-23 on lipid profile in SAPHO requires further study.
Navarro-Milán et al. [29] observed an increase in TC and LDL-C plasma levels in patients with early rheumatoid arthritis (RA) after MTX treatment. Marques et al. [30] reported that MTX treatment resulted in higher accumulation of lipids in adipocytes which can synthesize TG. Additionally MTX contributed to adipocyte hypertrophy and dysfunction, which could explain the increased MetS prevalence in RA patients treated with MTX [30]. In PsA patients after 24-month treatment with MTX, Costa et al. [31] found that the distribution of subjects affected by MetS at different time periods according to treatment was not modified over time, although they expected MetS components to be reduced after anti-inflammatory treatment. Our results are consistent with these data. We observed increased TG and LDL-C levels and increased prevalence of MetS in SAPHO patients treated with MTX. This could suggest that in SAPHO patients MTX treatment promotes or exacerbates MetS.
In our study, 86.6% of patients had increased tracer uptake on bone scintigraphy in sternoclavicular joints. This is consistent with data that we have previously published and with data presented by other authors [4, 7, 8, 22, 32].
There is not much known about the comorbidities in the course of SAPHO. Valkema et al. [33] observed a relatively high prevalence of autoimmune diseases in SAPHO patients. Sabugo et al. [34] presented a case of SAPHO and hypothyroidism that was successfully treated with infliximab, which interestingly after that treatment had decreased levothyroxine requirements [34]. We previously presented a case report of recurrent deep vein thrombosis caused by antiphospholipid syndrome in woman with SAPHO [35]. In our current study, the most common comorbidities were hypothyroidism and diabetes, and additionally 1 patient had Sjögren syndrome. This could confirm the suggestion of Valkema et al. [33] that autoimmunity may play a role in the pathomechanism of SAPHO syndrome.
We also observed a high prevalence of depression in our patients. We did not find data about increased risk of depression in SAPHO, but other authors reported increased risk of depression in patients with psoriasis and PsA [36, 37]. Moreover, Lewinson et al. [37] confirmed that depression increased risk of PsA in patients with psoriasis. Possibly the same relationship occurs in SAPHO.
No standard treatment protocols of SAPHO syndrome are available and current treatment options are not evidenced-based due to the rarity of the disease. Non-steroid anti-inflammatory drugs are considered to be the first-line agents in SAPHO, but in our study they were effective only in some patients, which is consistent with data from other studies [4, 19, 22].
Propionibacterium acnes is probably an important trigger of SAPHO syndrome, although it has only been found occasionally in bacterial cultures [11–13]. Antibiotics showed a good response in 39.1% of our patients, and antibiotics could be useful in the treatment of SAPHO syndrome [11–13].
We also observed that the use of disease-modifying antirheumatic drugs, particularly MTX and sulphasalazine, has been beneficial in some patients, although ineffective in others [5, 22].
In conclusion, serum interleukin-23 protects, whereas methotrexate treatment stimulates selected components of the MetS in patients with SAPHO syndrome. Increased prevalence of autoimmune diseases and depression was observed in SAPHO syndrome patients. Antibiotics could be useful in the treatment of patients with SAPHO. The novelty of this study is a comprehensive assessment of the impact of disease activity, selected serum cytokines, and therapy on MetS components in patients with SAPHO syndrome.