Introduction
Erectile dysfunction (ED) is a prevalent global issue which is defined as the persistent inability to achieve or maintain a rigid penile erection suitable for satisfactory sexual intercourse. The pathogenesis of ED is multifactorial, involving various factors, such as psychogenic (e.g., depression, anxiety, and stress), organic (e.g., aging, obesity, diabetes mellitus, and other conditions), or mixed causes [1]. ED may also be induced by unhealthy lifestyle factors, including smoking, alcohol consumption, insomnia, snoring, and lack of exercise [2, 3]. Numerous clinical studies have confirmed that adopting healthy lifestyle changes could serve as an effective strategy to mitigate the risk of ED [4–6].
Adverse sleep patterns have significant public health implications. Numerous studies have investigated the correlation between sleep traits, such as insomnia and obstructive sleep apnea (OSA), and the risk of ED [7–9]. A large cross-sectional observational study conducted in the Chinese population supports the findings of a Taiwanese population-based cohort study. The study revealed that patients with OSA had a significantly higher incidence of ED [10, 11]. During a 3-year follow-up period involving 539,109 men with insomnia, both untreated and treated insomnia were associated with an increased risk of ED [7]. The results of monitoring sleep quality in patients with ED showed that both total sleep time and duration of deep sleep were significantly lower than those in the non-ED group [12]. However, traditional observational studies are greatly influenced by factors such as sample size, confounding variables, and reverse causation.
The Mendelian randomization (MR) study is a research technique that investigates the causal relationship between exposure and outcome, similar to that of randomized controlled trials [13]. Single-nucleotide polymorphisms (SNPs) that exhibited a strong correlation with exposure were used as instrumental variables (IVs) to determine the presence of a causal relationship between exposure and outcome in MR studies.
The objective of this study was to evaluate the causal relationship between sleep traits and ED using a two-sample MR study.
Material and methods
Study design overview
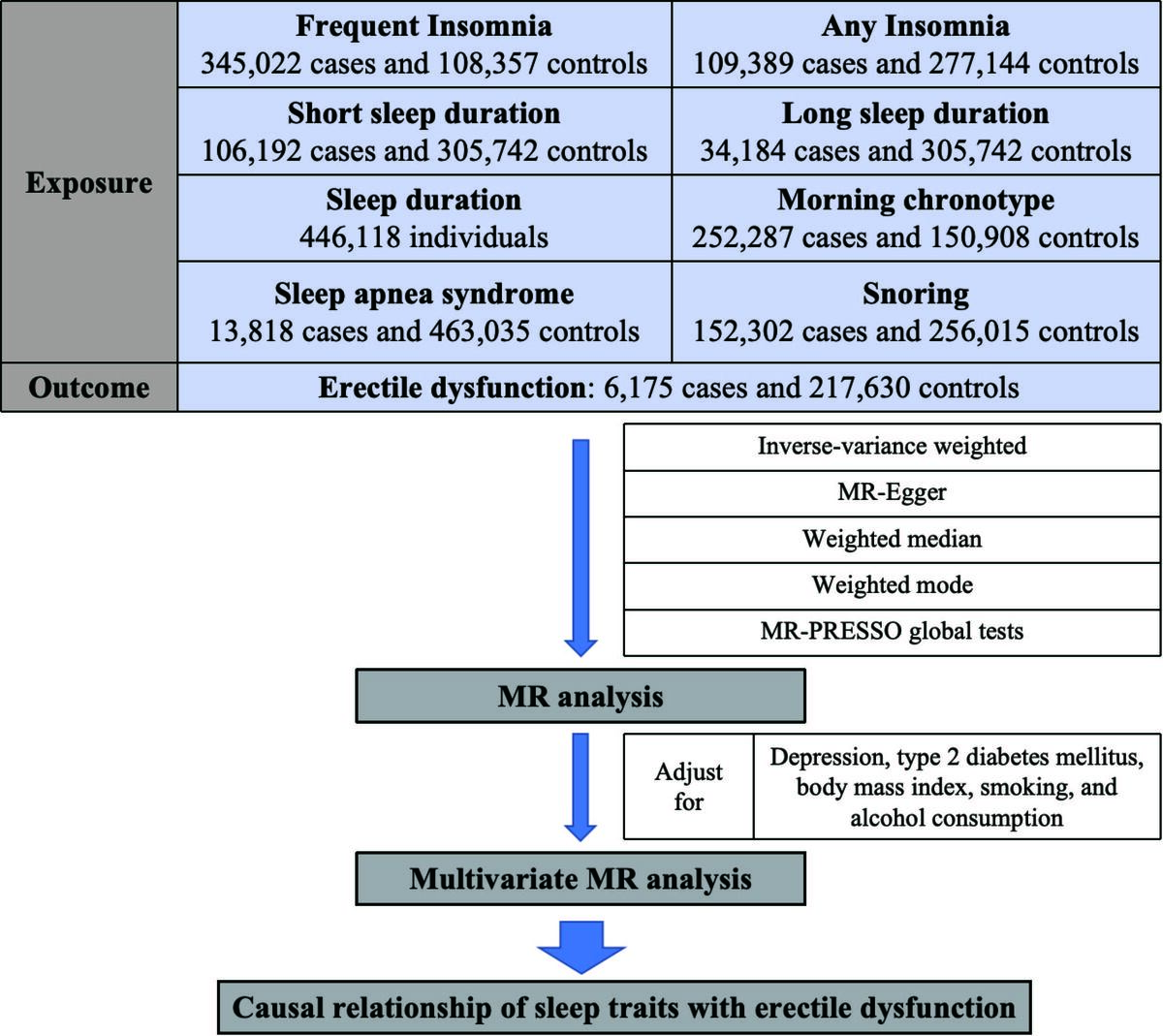
We conducted a two-sample MR analysis using publicly available data from genome-wide association studies (GWAS). This study included sleep traits, such as insomnia, sleep duration, morning chronotype, snoring, and OSA. The MR approach relies on three crucial assumptions: (1) the IVs should have a strong association with exposure; (2) the IVs should not be associated with any confounding factors; and (3) the IVs should only influence the outcome through exposure.
Data sources for sleep traits, ED, and potential confounders
Sleep duration [14]: Summary-level information on sleep duration, including short sleep (≤ 6 h/day) and long sleep (≥ 9 h/day), was acquired from the GWAS data of 446,118 individuals of European ancestry. The data analysis included 106,192 cases with short sleep duration and 305,742 controls, as well as 34,184 cases with long sleep duration and 305,742 controls (Table I).
Table I
Detailed information on traits included in this study
Insomnia: Summary-level information on insomnia was acquired from GWAS data of 453,379 individuals of European ancestry [15] (345,022 cases with frequent insomnia and 108,357 controls) and GWAS data of 1,331,010 individuals of European ancestry [16] (109,389 cases with any insomnia and 277,144 controls) (Table I).
Morning person [15]: Summary-level information on morning chronotypes was acquired from the GWAS data of 449,734 individuals of European ancestry. This dataset included 252,287 cases of morning chronotypes and 150,908 controls (Table I).
Snoring [17]: Summary-level information on snoring was acquired from the GWAS data of 408,317 individuals of European ancestry. Within this dataset, 152,302 snoring cases and 256,015 controls were included (Table I).
Sleep apnea syndrome [18]: Summary-level information on sleep apnea syndrome was acquired from the GWAS data of 476,853 individuals of European ancestry. This dataset included 13,818 patients with sleep apnea symptoms and 463,035 healthy controls (Table I).
ED [19]: Summary-level information on ED was acquired from the GWAS data of 223,805 individuals of European ancestry. The study included 6,175 patients with ED and 217,630 controls (Table I).
Potential confounders: To account for potential genetic confounders associated with sleep traits, such as depression [20], type 2 diabetes mellitus (T2DM) [21], body mass index (BMI) [22], smoking [23], and alcohol consumption [23], we investigated the direct effect of certain sleep traits on ED using a multivariate MR approach by adjusting for these factors. Summary-level information on these factors is provided in Table I.
Further information on the diagnostic criteria for exposures and outcomes can be obtained from the abovementioned literature.
Selection of instrumental variables
In the first MR analysis, IVs were used based on a genome-wide significance threshold (p < 5e−8) to estimate the causal effect of sleep traits on ED risk. In the second MR analysis, IVs screened using a locus-wide significance threshold (p < 1e−5) were evaluated to determine the causal effect of sleep traits on ED risk. SNPs within a 10,000 kb window were eliminated based on a threshold of r2 < 0.001, to alleviate the effects of linkage disequilibrium (LD). Then, the strength of the IVs was assessed using F-statistics, which were calculated according to the formula F = R2 × (N – 2)/(1 – R2), where R2 represents the proportion of variance explained and N is the total sample size. The following formula was used to compute R2: 2 × EAF × (1 – EAF) × β2, where EAF denotes the effect allele frequency, and β represents the estimated genetic effect on exposure risk. IVs with F-statistics > 10 were commonly chosen to reduce the potential bias from weak IVs.
Statistical analysis
This study used four different MR analysis methods: inverse-variance weighted (IVW) [24], MR-Egger [25], weighted median [26], and weighted mode [27]. The IVW method was primarily used for the meta-analysis of the SNP-specific Wald estimates, assuming balanced pleiotropy. The outcomes of the causal relationships were expressed in terms of odds ratios (OR) and 95% confidence intervals (95% CI).
Several sensitivity analyses were also performed. First, the significance of the MR-Steiger test results suggested that causal inference was not biased by reverse causation. Otherwise, this indicated the presence of a reverse causal direction. Next, Cochran’s Q test was used to assess heterogeneity. A p-value of < 0.05 indicated significant heterogeneity. The random-effects IVW model was used in subsequent analyses. Otherwise, a fixed-effects IVW model was used. Then, the MR-Egger intercept and MR-PRESSO global tests were used to detect any potential pleiotropic effects of the genetic variants on the estimate of causality. Finally, leave-one-out analysis was performed by removing each IV to determine whether the results were disproportionately affected by a single SNP. In multivariate MR analyses, direct causal effects were estimated using the IVW method after adjusting for depression, T2DM, BMI, smoking, and alcohol consumption. The statistical analyses were conducted using R software (v 4.2.1). The MR analysis was conducted using the R packages “Two Sample MR,” “Mendelian Randomization,” and “’MRPRESSO.”
To minimize the occurrence of false positives due to multiple comparisons (eight exposure factors), we used the Benjamini-Hochberg false discovery rate (FDR) correction. P-values that passed a critical value corresponding to an FDR of 0.05 were considered strong evidence of associations. P-values that did not pass a critical value but were less than 0.05 were considered suggestive evidence of associations.
Results
Results of MR analysis using IVs screened based on genome-wide significance (p < 5e−8)
MR analysis revealed that increased snoring was associated with an elevated risk of ED (IVW: OR = 4.16, 95% CI: 1.40–12.38, p = 1.04e-2; PFDR = 4.99e-2) (Supplementary Figure S1). Furthermore, we found that being a morning person was associated with a lower risk of developing ED (IVW: OR = 0.86, 95% CI: 0.77–0.97, p = 1.25e-2; PFDR = 4.99e-2). However, the results indicated no evidence supporting a potential causal effect of short sleep, long sleep, sleep duration, sleep apnea syndrome, any insomnia, or frequent insomnia on the risk of ED according to the IVW analysis (all p > 0.05).
For the sensitivity analysis, Supplementary Figures S2–S4 depict funnel plots, scatter plots, and results of the leave-one-out analysis. The MR-Steiger test results suggested no bias in causal inference due to reverse causation (p < 0.05). No heterogeneity was found in the MR analysis, as determined by Cochran’s Q test (p > 0.05). The MR-Egger intercept test and MR-PRESSO global test indicated that MR analysis was not affected by horizontal pleiotropy (p > 0.05) (Supplementary Table SI).
Results of MR analysis using IVs screened based on locus-wide significance (p < 1e−5)
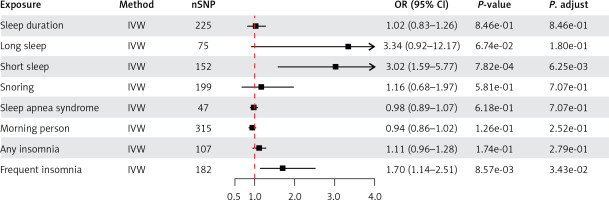
MR analysis revealed that a shorter sleep duration was associated with a higher risk of ED (IVW: OR = 3.02, 95% CI: 1.59–5.77, p = 7.82e-4; PFDR = 6.25e-4) (Figure 1). Furthermore, we found that frequent insomnia was a risk factor for ED (IVW: OR = 1.70, 95% CI: 1.14–2.51, p = 8.57e-3; PFDR = 3.43e-2). However, the results showed no evidence of a potential causal effect of long sleep, sleep duration, sleep apnea syndrome, morning person, any insomnia, or frequent insomnia on the risk of ED according to the IVW analysis (all p > 0.05).
Figure 1
Causal effects of sleep traits on ED in univariable MR analysis using IVs screened based on the locus-wide significance
MR – Mendelian randomization, IVW – inverse variance weighted, CI – confidence interval, OR – odds ratio, SNP – single nucleotide polymorphism, ED – erectile dysfunction.
Supplementary Figures S5–S7 depict funnel plots, scatter plots, and leave-one-out analysis results for the sensitivity analysis. The significance of the MR-Steiger test results suggested that there was no bias in causal inference due to reverse causation (p < 0.05). No heterogeneity was found in the MR analysis, as determined by Cochran’s Q test (p > 0.05). The MR-Egger intercept test and MR-PRESSO global test indicated that MR analysis was not affected by horizontal pleiotropy (p > 0.05) (Table II).
Table II
Sensitivity analysis of MR analysis using IVs screened based on locus-wide significance (p < 1e-5)
Results of multivariable Mendelian randomization analysis
We conducted multivariate MR analysis to evaluate the direct causal effect of short sleep duration and frequent insomnia on the risk of ED. This analysis considered five confounding factors: depression, BMI, smoking initiation, alcohol consumption, and T2DM (Figure 2). Multivariable MR analysis revealed that after adjusting for smoking (OR = 2.68, 95% CI: 1.23–5.84, p = 1.30e−02), alcohol consumption (OR = 2.82, 95% CI: 1.39-5.71, p = 4.09e−03), BMI (OR = 3.74, 95% CI: 1.54–9.11, p = 3.64e−03), and T2DM (OR = 2.95, 95% CI: 1.19–7.31, p = 1.91e−02), short sleep duration remained causally related to the risk of ED. After adjusting for smoking (OR = 2.19, 95% CI: 1.42–3.39, p = 4.07e−04), alcohol consumption (OR = 2.14, 95% CI: 1.41–3.26, p = 3.60e−04), depression (OR = 1.76, 95% CI: 1.05–2.94, p = 3.21e−02), and T2DM (OR = 2.28, 95% CI: 1.34–3.90, p = 2.53e−03), frequent insomnia remained causally related to the risk of ED. Interestingly, after adjusting for depression for short sleep (OR = 2.05, 95% CI: 0.94–4.46, p = 7.03e−02) and after adjusting for BMI for frequent insomnia (OR = 1.66, 95% CI: 0.96–2.87, p = 7.19e−02), the causal relationship between these two sleep traits and ED risk was no longer present.
Discussion
To the best of our knowledge, this is the first study to comprehensively integrate the majority of sleep traits relevant to clinical practice and use multiple MR analysis to investigate the causal relationship between sleep traits and ED. Given the fact that ED patients often have poor sleep quality, it is currently inconclusive whether ED causes poor sleep quality or poor sleep quality ultimately leads to ED. Our initial MR results demonstrated a causal relationship between morning chronotype and a lower risk of ED. Additionally, snoring was causally associated with a higher risk of ED. In contrast, our second set of MR results demonstrated that both short sleep duration and frequent insomnia were causally associated with a high risk of ED. Although the results of these two MR analyses were inconsistent, the causal relationships between sleep traits and ED were robust, as indicated by the sensitivity analysis. Furthermore, the association persisted in the multivariate analyses after adjusting for potential confounding factors. Meanwhile, our research found no reverse causal relationship between sleep disorders and ED. Notably, after depression adjustment for short sleep and BMI adjustment for frequent insomnia, no causal association was found between these two sleep traits and ED risk.
Sleep is an essential internal activity for human health. Sleep disorders have been linked to various health problems, such as cardiovascular disease, obesity, mental health, and neurodegenerative diseases. Many of these conditions share common causes with ED [28]. Furthermore, medical interventions for sleep disorders, which often act through central nervous system pathways and include sedatives, may also impact ED [7]. Insomnia is a major form of sleep disorder characterized by difficulty in falling asleep, reduced sleep quality and duration, and impaired memory and concentration. Insomnia has been suggested as a potential risk factor for ED [7, 29]. Based on genetic data, our findings align with those of Xiong et al. [30], indicating that insomnia increases the risk of ED. In contrast, other studies have failed to find a relationship between insomnia and ED [31, 32]. In their study, insomnia did not increase the risk of ED.
Snoring is another form of sleep disorder. A previous study provided genetic evidence that snoring, a feature of OSA, increases the risk of ED [33]. The relationship between OSA and ED has been extensively studied for several decades. However, there is conflicting evidence linking OSA with ED. Some studies have reported that OSA is not associated with ED [34, 35]. Another study reported a strong correlation between the severity of OSA and decreased sexual satisfaction but no significant association with ED [8]. Continuous positive airway pressure (CPAP) and surgical treatments were demonstrated as effective therapies for OSA. It has been proved that CPAP therapy significantly improves erectile function of OSA patients, and a combined treatment with sildenafil provides a cumulative effect [36]. In addition, a large and long-term cohort study which included 11,116 OSA patients showed that surgical treatments for OSA could reduce the risk of developing ED by 21% [37]. In our research, we have not identified genetic evidence of a causal relationship between OSA and ED.
Short sleep is also a form of sleep disorder. In modern society, the primary causes of short sleep among the public include shift work schedules, staying up late or developing late sleeping habits, and engaging in various nighttime activities that disrupt sleep. According to Rodriguez et al. [38], men who work shifts, particularly night shifts, exhibit lower erectile function. This could be attributed to inadequate sleep and disrupted circadian rhythm. However, most studies have focused on investigating OSA and insomnia-related sleep disorders. Short sleep, similar to other sleep disorders, is equally important for meeting the social value requirements for clinical research [39].
Clinical observational studies have found that the association between sleep disorders and ED is unclear, primarily because of the presence of confounding factors. However, further research is needed to determine whether sleep disorders are risk factors for ED or whether they are comorbid symptoms of ED. Furthermore, there may be interactions or coexistence between different types of sleep disorders, making the establishment of a clear relationship between a specific type of sleep disorder and ED challenging. Although the specific mechanism by which sleep disorders contribute to ED is not yet fully understood, the prevailing belief is that the hypothalamic-pituitary-gonadal axis may have a significant effect on this phenomenon. In a 10-year follow-up cohort study that included 3314 participants, sleep disorders were found to disrupt the circadian rhythm of cortisol secretion. Individuals with sleep disorders showed a sharper surge in morning cortisol levels and higher cortisol levels later in the day than the control group [40]. According to the findings of the largest male patient cohort study to date, men diagnosed with circadian rhythm dysfunction, insomnia, or sleep apnea had higher risks of testosterone deficiency and ED [41]. Similarly, in a recent study by Rodriguez et al. [38], individuals with shift work sleep disorders were found to have lower testosterone levels compared to matched controls. Notably, testosterone therapy could partly reverse the effects of shift work sleep disorders and improve erectile function. Overall, most studies suggest that sleep disorders are associated with lower testosterone levels, which play an important role in the male erectile process. However, it was also observed that hypoxia may develop from sleep disorders [42]. It has been demonstrated that hypoxia can induce oxidative stress damage and downregulate the NO/cGMP signaling in sleep-deprived rat models [43]. Furthermore, sleep disorders and ED share common risk factors, such as mental disorders, obesity, hypertension, diabetes, and metabolic syndrome. These conditions also contribute to the development of ED.
This study has several highlights. First, previous research has focused only on one or a few sleep traits. In our study, we included a wide range of sleep traits commonly observed in clinical practice. We used univariate and multivariate MR methods to explore the causal relationship between these sleep traits and ED. Second, the present MR analysis was conducted using separate summary-level data from a large-scale GWAS, which enhanced the confidence of the inference due to the significant sample size. Third, the reliability of the results was improved by using various MR techniques and conducting sensitivity analyses.
However, this study has some limitations. First, the conclusions of this study may only be applicable to Europeans, since the original GWAS summary-level data used in the analysis were sourced from European populations. Second, there may be some overlap between different sleep traits or between sleep traits and ED, which could result in overfitting and undermining causal inference. Finally, the diagnosis of sleep traits and ED was largely based on questionnaires, which may have introduced subjectivity. Moreover, the pharmacological treatment of sleep disorders may have a negative impact on erectile function, thereby influencing the accuracy of research findings. In the future, the use of multiple objective measures is advisable to diagnose sleep traits and ED to gain a better understanding of the relationship between sleep traits and ED outcomes.
In conclusion, this study strengthens the evidence that genetically predicted snoring and insomnia are associated with an increased risk of developing ED. Additionally, the study highlights the causal relationship between ED and short sleep duration, as well as chronotype. Employing more advanced analytical methods and utilizing updated GWAS data can enhance the accuracy and validity of our findings.