Introduction
In children, nephrotic syndrome (NS) is one of the most common glomerular diseases, with an incidence of 4.7 (range: 1.15–16.9) per 100,000 children worldwide [1]. Primary, or idiopathic, NS is commonly seen in 95% of patients [2]. Eighty to 90% of patients respond to prednisolone as a steroid-sensitive nephrotic syndrome (SSNS) [3]. Furthermore, 80–90% of SSNS children experience one or more relapses [4, 5]. Approximately 50% of these relapsing children develop steroid-dependent nephrotic syndrome (SDNS), a condition with an increased risk of progressing to end-stage renal disease [6].
Corticosteroids can cause significant adverse effects when used long term. As steroid-sparing agents, immunosuppressive drugs such as calcineurin inhibitors (CNIs), mycophenolate mofetil (MMF), and cytotoxic agents are used [7], and the results appear promising. Meanwhile, some of these immunosuppressants can have serious adverse effects, such as nephrotoxicity, hyperglycemia, headaches, dyslipidemia, and weakened immune function [8]. Novel drugs are needed to address these problems.
Rituximab (RTX) is a monoclonal antibody that acts directly against CD20 expressed on B lymphocytes. It is now increasingly used in various autoimmune diseases [9]. Recently, RTX was introduced for the treatment of relapsing NS [10, 11]. However, the efficacy and safety of RTX in this treatment remain controversial. We aimed to evaluate both the effectiveness and safety of RTX in the treatment of children with SDNS or FRNS via a meta-analysis (PROSPERO registration number: CRD42021293915. INPLASY registration number: INPLASY2021110099).
Material and methods
Data sources and research
This meta-analysis was conducted according to the recommendations of the PRISMA guidelines [12]. The meta-analysis searches were conducted using the PubMed, Embase, Web of Science, and Cochrane Library databases from their inception date to November 30th, 2021. Two investigators independently performed a systematic search using medical subject headings (MeSH) terms for Medline, EMTREE terms for Embase, and free words to search for “Rituximab”, “CD20”, “CD20 Antibody”, “Children”, and “Nephrotic Syndrome,” and additionally we performed backtracking searches for references of related literature.
Study selection and data collection process
The study type was restricted to randomized controlled trials (RCTs). FRNS or SDNS children younger than 18 years of age were included. FRNS is defined as two or more relapses within 6 months after first remission, or four or more relapses within any 12-month period. SDNS is defined as two consecutive relapses during prednisolone dose reduction or within 2 weeks after its discontinuation. Children who received any dose of RTX were enrolled in the RTX group. All others were assigned to the control group, having received conventional drugs, such as steroids and/or CNIs, MMF, cyclophosphamide and placebo.
Two independent reviewers evaluated the references obtained from the electronic search. There were no restrictions by language of publication. The initial assessment was based on screening titles and abstracts. Studies that were not excluded after an initial evaluation were retrieved for full-text analysis. The final decision was made by consensus among the authors in cases of disagreement. Review articles, case reports, comments, meeting abstracts, editorials, and studies containing both pediatric and adult populations without subgroup analysis were excluded.
The data were extracted by two independent reviewers. The extracted data included first author, publication year, country, sample size, age of children, sex, interventions and controls, diagnosis, follow-up duration, and outcome measures. The patients’ outcomes comprised short-term (3 to 6 months) and long-term (12 months) complete remission, steroid dosage, serum albumin, proteinuria, eGFR, height Z score, BMI Z score, and adverse events. The extracted data were mostly represented by the mean and standard deviation (SD).
Bias and quality assessments
The Cochrane Risk of Bias tool was used to assess the risk of bias. Two reviewers labelled each trial as “low”, “unclear”, or “high” risk of bias. If at least one key domain was judged to be at high risk, it was considered at high risk of bias overall. If all key domains were judged to be low risk, it was considered low risk of bias. Otherwise, it was considered as an unclear risk of bias.
Statistical analysis
Relative risks (RRs) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes. Standardized mean differences (SMDs) and mean differences (MDs) with 95% CIs were calculated for continuous outcomes. Statistical heterogeneity was evaluated using the I2 statistic and the Cochrane Q statistic. Data were analyzed with a random-effects model if I2 > 50% or p < 0.10; otherwise, a fixed-effects model was used. A predefined subgroup analysis was performed when heterogeneity was high, and a sensitivity analysis was conducted by omitting each trial in turn to explore potential sources of heterogeneity. Funnel plots and Egger’s test were conducted to evaluate publication bias. Review Manager 5.3 and STATA 15.0 (StataCorp, College Station, TX) were used to perform the statistical analyses.
Results
Search flow and description of included studies
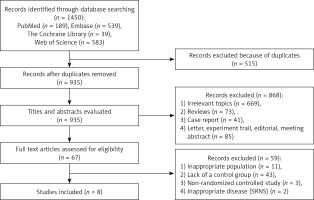
1450 studies were identified in the initial literature search. We excluded repetitions, reviews, nonrandomized controlled studies and studies inconsistent with the purpose of evaluation. Finally, eight studies [13–20] eligible for inclusion criteria were included in our study, and the screening flow chart is shown in Figure 1.
Characteristics of included studies
There were 476 patients in the eight studies, ranging from 23 to 120. The average age of all patients was approximately 9.53 ±10.25 years. All studies mentioned the follow-up duration of the disease: from 6 months to 5 years. Of the eight included studies, three were conducted in Italy, two in India, one in France, one in Japan, and one in Korea. Among them, five studies compared the effects of RTX with placebo or conventional treatment strategies, including prednisolone and/or CNIs, MMF, or cyclophosphamide. Two studies included patients who had not received tacrolimus before enrollment and compared the effects of RTX with tacrolimus. One study included patients with only low-dose prednisone (0.1–0.4 mg/kg/day) and compared whether RTX is noninferior to steroids in maintaining SDNS in remission in children treated with low-dose prednisone. The basic characteristics of the included studies are listed in Table I. The interventions of the RTX group and the control group are described in Supplementary Table SI.
Table I
Characteristics of studies included in the meta-analysis
| Studies | Country | Study design | Group | Cases | Age [years] | Sex (male/female) | Weight [kg] | Duration of NS [years] | Serum albumin [g/dl] | eGFR [ml/min/1.73 m2] | Dose of prednisone [mg/kg/day] | Follow-up [months] | Histology RTX group | Histology control group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn YH 2018 [13] | Korea | Open-label RCT | RTX Control | 35/16 | 13.5 ±5.0 12.5 ±4.2 | 26/9 13/3 | NA | 8.7 ±4.9 7.4 ±4.9 | NA | 115.1 ±32.7 112.6 ±35.9 | 0.456 ±0.399 0.321 ±0.331 | 12 | MCD: 23 FSGS: 2 Unknown: 10 | MCD: 8 FSGS: 1 Unknown: 7 |
| Basu B 2018 [14] | India | Parallel-arm, open-label RCT | RTX Control | 60/60 | 7.1 ±2.8 7.2 ±2.8 | 32/28 32/28 | 27.5 ±8.6 27.8 ±8.8 | 2.3 ±1.7 2.5 ±1.5 | 4.18 ±0.73 4.34 ±0.81 | 100.2 ±8.6 103.0 ±10.8 | 1.3 ±0.2 1.3 ±0.2 | 12 | MCD: 43 FSGS: 17 | MCD: 42 FSGS: 18 |
| Boumediene A 2017 [15] | France | Double-blind RCT | RTX Control | 10/13 | 11.1 ±1.2 12.3 ±1.0 | 10/0 6/7 | NA | NA | NA | NA | NA | 6 | MCD: 9 Unknown: 1 | MCD: 11 FSGS: 1 Unknown: 1 |
| Iijima K 2014 [16] | Japan | Double-blind RCT | RTX Control | 24/24 | 11.5 ±5.0 13.6 ±6.9 | 18/6 16/8 | 44.0 ±18.6 47.5 ±15.6 | 7.9 ±4.7 8.0 ±5.4 | 3.4 ±0.6 3.4 ±0.5 | 128.9 ±20.6 126.4 ±26.0 | (19.13 ±9.94) mg/m2/day (18.02 ±10.15) mg/m2/day | 12 | MCD: 21 FSGS: 2 Unknown: 1 | MCD: 23 FSGS: 1 |
| Ravani P 2011* [17] | Italy | Open-label RCT | RTX Control | 27/27 | 10.2 ±4.0 11.3 ±4.3 | 24/3 19/8 | 39.6 ±15.2 45.5 ±19.3 | 5.7 ±3.5 7.8 ±4.0 | 3.6 ±0.9 3.2 ±0.8 | NA | 0.57 ±0.42 0.60 ±0.47 | 12 | MCD: 13 FSGS: 7 Unknown: 7 | MCD: 6 FSGS: 10 Unknown: 11 |
| Ravani P 2015* [18] | Italy | Open-label, non-inferiority RCT | RTX Control | 15/15 | 6.9 ±3.6 6.9 ±3.1 | 10/5 11/4 | 30 ±16 31 ±14 | 2.7 ±2.4 2.0 ±2.5 | NA | NA | 0.57 ±0.42 0.60 ±0.47 | 12 | NA | NA |
| Ravani P 2020* [19] | Italy | Open-label RCT | RTX Control | 15/15 | 7.4 ±2.6 6.0 ±3.6 | 9/6 12/3 | 30 ±12 25 ±14 | 2.5 ±1.9 1.6 ±2.2 | 3.5 ±0.6 3.5 ±0.5 | NA | 0.7 ±0.3 0.8 ±0.4 | 12 | NA | NA |
| Solomon N 2019 [20] | India | Open-label, parallel-arm RCT | RTX Control | 60/60 | NA | NA | NA | NA | NA | NA | 0.456 ±0.399 0.321 ±0.331 | 12 | NA | NA |
Quality assessments
The risk of bias assessment is shown in Supplementary Figure S1. All studies mentioned randomization, but only six studies had a detailed description of random sequence generation. Seven studies described allocation concealment, and only two studies adopted the blind method. All studies mentioned follow-up and described the drop-out or withdrawal information.
Short-term complete remission rate (3 to 6 months)
In the included studies, seven reported a complete remission rate within the short term (3 to 6 months). Funnel plots and Egger’s test indicated the existence of publication bias in the 7 studies (p < 0.001) (Supplementary Figure S3).
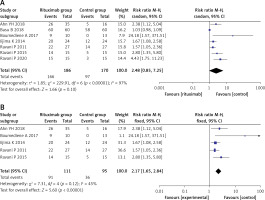
We found significant heterogeneity in the short-term complete remission rate, with I2 = 97%, p < 0.001 (Figure 2 A). After sensitivity analysis, we found that Basu’s study [14] and Ravani’s study in 2020 [19] could be sources of heterogeneity (Supplementary Figure S2). When these two studies were excluded, the results showed that the RTX group had a significantly increased short-term complete remission rate (RR = 2.17, 95% CI: 1.65, 2.84, p < 0.001]. The results on short-term complete remission rate are presented in Figure 2 B.
Long-term complete remission rate (approximately 12 months)
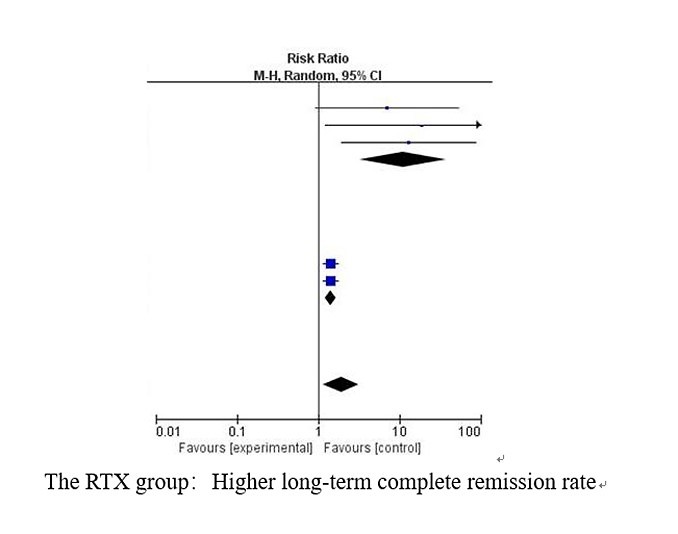
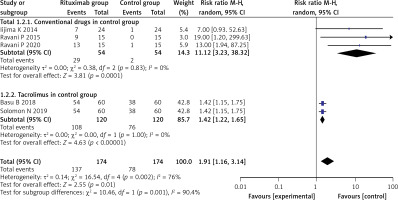
Five studies reported the relapse number in the RTX group and the control group after corresponding treatment in the long term (approximately 12 months). We found significant heterogeneity in the long-term complete remission rate (I2 = 76%, p = 0.002). After sensitivity analysis, we found that Basu’s study [14] and Solomon’s study [20] could be sources of heterogeneity. We conducted subgroup analysis to compare the long-term complete remission rate of the RTX group with the conventional treatment strategy and tacrolimus. The results showed that the RTX group had a significantly higher long-term complete remission rate compared with both the conventional treatment strategy and tacrolimus, whose RRs (95% CI) were (11.12 (3.23, 38.32), p < 0.001)) and (1.42 (1.22, 1.65), p < 0.001), respectively. No obvious heterogeneity was found in either subgroup (I2 = 0%, p = 0.83; I2 = 0%, p = 1.00). These results are shown in Figure 3.
Long-term complete remission rate (> 12 months)
Two studies had a follow-up duration of more than 12 months. Ravani’s study in 2015 [18] enrolled SDNS children maintained in remission with high prednisone doses (≥ 0.7 mg/kg/day) and reported that 40% were still in remission at 60 months in the RTX group, with a median relapse-free time of 18 months in the rituximab group. 46.7% of children had received one to three rituximab courses. Ravani’s study in 2020 [19] enrolled SDNS children maintained in remission with low prednisone doses (0.1–0.4 mg/kg/day) and found that 53% of patients who had a single intravenous infusion of rituximab were still in remission after 4-year follow-up compared to a 40% remission rate in the control group.
Proteinuria
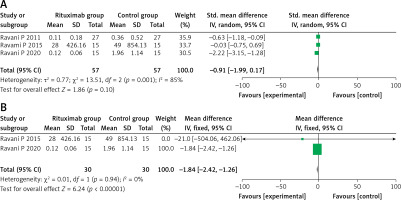
Three studies evaluated the results of proteinuria level, among which Ravani’s study in 2011 [17] used a different unit of measurement (g/day) than the other two studies (mg/m2/day). There was significant heterogeneity among the three studies (SMD = –0.91, 95% CI: –1.99, 0.17, p = 0.10; I2 = 85%, p < 0.01), as shown in Figure 4 A. When we excluded Ravani’s study in 2011 [17], no obvious heterogeneity was seen between the other two studies (MD = –1.84, 95% CI: –2.42, –1.26, p < 0.001; I2 = 0%, p = 0.94), as shown in Figure 4 B. The two studies showed the RTX group to have a better improvement in reducing proteinuria levels.
Steroid dosage
Five studies evaluated prednisolone dose after treatment. A significant difference between the RTX group and the control group was found (SMD = −0.87, 95% CI: –1.32, –0.43, p < 0.001) (Supplementary Figure S4 A). However, there was obvious heterogeneity among these studies (I2 = 67%, p = 0.02), which might be a result of discordance of the units of steroid dosage. When Iijima’s study [16] was excluded, the four remaining studies did not show obvious heterogeneity (I2 = 42%, p = 0.16) (Supplementary Figure S4 B). The results indicated that RTX decreased the dose of prednisolone used compared with the control group.
Serum albumin
Two studies evaluated the results of serum albumin using different units of measurement (SMD = 1.25, 95% CI: 0.37, 2.13, p < 0.01; I2 = 80%, p = 0.02) (Supplementary Figure S5). Compared with the control group, the RTX group had a higher value of serum albumin.
eGFR
Two studies evaluated the results of estimated renal function using eGFR (MD = 6.51, 95% CI: 2.70, 10.32, p < 0.001; I2 = 0%, p = 0.86) (Supplementary Figure S6). Compared with the control group, the RTX group had a higher eGFR value.
Height Z score and BMI Z score
Four studies evaluated the results of the height Z score (MD = 0.19, 95% CI: 0.01–0.37, p = 0.04; I2 = 0%, p = 0.64) (Supplementary Figure 7 A). Compared with the control group, the RTX group had higher values for children’s growth data. Two studies evaluated the results of BMI Z score (MD = –0.10, 95% CI: –0.36, 0.16, p = 0.44; I2 = 42%, p = 0.19) (Supplementary Figure 7 B). From the results of this analysis, there was no significant difference in BMI Z score between the RTX group and the control group.
Adverse events
Infusion reactions
Three studies reported the results of infusion reaction events (RR = 8.18, 95% CI: 0.25–263.28, p = 0.24; I2 = 89%, p < 0.001) (Supplementary Figure 8 A). No significant differences were observed in the occurrence rate of infusion reactions between the two groups.
Discussion
The meta-analysis included eight studies with 476 patients, including 246 patients in the RTX group and 230 patients in the control group. Our study indicated that RTX treatment was effective both in the short term (3 to 6 months) and long term (12 months) in improving the relapse-free rate of childhood SDNS/FRNS. Furthermore, RTX treatment reduced proteinuria and the dose of prednisolone used, which was able to significantly increase the eGFR and children’s growth data. There were no significant differences in adverse events.
There have also been three similar meta-analyses about RTX treatment in NS [21–23] recently. Gao’s study [21] and Chang’s study [23] included both SDNS and refractory steroid-resistant nephrotic syndrome (SRNS) (Magnasco’s study [24] cited in Gao’s study [21]). Liu’s study [22] included both RCTs and a control study (Sinha’s study [25]). Recent studies showed that RTX might be more effective in maintaining remission of proteinuria in children with SDNS than in SRNS [26],while others found no improvement of proteinuria in SRNS following rituximab therapy [24]. Considering the differences of therapeutic effect between the two different clinical types, we only focused on the effect of RTX treatment on SDNS/FRNS. Unlike Liu’s study [22], our study focused specifically on RTX treatment in SDNS or FRNS via high quality RCTs to assess the efficacy and safety, and discuss future perspectives.
We found that the RTX group experienced a significant reduction in relapses of NS compared with the control group in short-term complete remission. The significant heterogeneity in the short-term complete remission rate may be partly due to the severity of the diseases included in different studies. Grading the severity of disease in the enrolled patients in the design allows for a more rigorous judgement of the efficacy of RTX.
In our study, the RTX group showed a significant increase in the long-term (12 months) complete remission rate. The significant heterogeneity in the complete remission rate may be partly due to different control groups in Basu’s study [14] and Solomon’s study [20], which chose patients who received tacrolimus as a control group to compare the effects of RTX. RTX offers an alternative to current immunosuppressive therapies for difficult-to-treat NS. The best outcomes are seen in patients with SDNS who failed to respond to multiple therapies. Hofstra et al. [27] found that proteinuria decreased significantly (by 2–3 g/day) within 2 weeks of RTX infusion. In addition, Gulati et al. [26] found that 82% and 40% of SDNS patients had reduced proteinuria and normalized serum albumin after RTX was administered at the end of a 4.5-month study follow-up, respectively. Sergeeva et al. [28] reported that steroids were discontinued in 50% of patients after 6 months of initial treatment with RTX and in 64% at 12 months, and reported a significant reduction in the dose of prednisolone in other participants who remained dependent on high doses of prednisolone during inclusion. With a decrease in the dose of prednisolone, the inhibition of height decreased, which is understandable. Wang et al. [29] found that at 1 year after treatment, the RTX group had higher total, psychological health summary, and social and school functioning scores than the cyclophosphamide and tacrolimus groups. Indeed, these data show that RTX treatment can be effective in facilitating the remission and recovery of the disease.
However, data on the long-term complete remission rate (> 12 months) are still limited. Kim et al. [30] conducted a 5-year long-term retrospective study and found that after receiving 5.2 ±2.3 cycles of RTX treatment, the relapse rate decreased from 3.4 ±2.0 per year initially to 0.4 ±0.8 per year in the third year, which indicated that preemptive and long-term, repeated RTX treatment is relatively effective. Two studies in our study mentioned the long-term (> 12 months) complete remission rate. 46.7% of children in Ravani’s study from 2015 [18] received one to three rituximab courses with an encouraging result. The subjects of Ravani’s study in 2020 [19] were SDNS children maintained in remission with low prednisone doses (0.1–0.4 mg/kg/day), who improved after single intravenous infusion of rituximab. Therefore, the proportion of patients who achieved sustained remission depended on the dosing strategy and duration of follow-up [31, 32]. Most patients experienced a relapse after B cell recovery, which might require long-term immunosuppressive therapy or additional doses of RTX. It is still worth exploring whether RTX can be tapered, discontinued, or, on the other hand, even used lifelong. A consensus on the optimal dosage, interval of RTX administration in NS, specific treatment regimen and long-term follow-up is urgently needed.
Our study shows that most patients tolerated RTX treatment well. Subun et al. [33] found that RTX seems to be safe even after several repeated courses. Webb et al. [34] reported that RTX was associated with fewer side effects than was cyclophosphamide, with only allergic reactions at infusion in 2 patients.
Although the role of RTX in the treatment of NS has received widespread attention, there is still no detailed consensus on the optimal regimen. One of the major concerns is reduction of immune function, such as severe neutropenia and persistent hypogammaglobulinemia [35, 36]. Furthermore, patients may not maintain long-term remission following B-cell recovery, possibly due to the development of autoreactive long-lived plasma cells. Apart from this, some rare but serious side effects, such as RTX-induced serum sickness and anti-RTX antibodies (ARAs), might occur after long-term RTX use [37–39]. More studies are needed to further explore the impact of RTX treatment on the body’s immunity and its long-term side effects.
Our study has some limitations. Insufficient data were available due to the limited number of clinical studies in the pediatric population and the short application history of RTX. Also, different RTX therapy regimens were used in the studies, including dose of RTX, number of infusions, and maintenance immunosuppression. In addition, there was publication bias in this meta-analysis, but we could not reliably assess for it as there were too few studies (< 10 trials). Furthermore, studies included in our meta-analysis enrolled patients from different regions and various ethnic groups, which may introduce differences in basic characteristic among these patients. Long-term follow-up and additional well-designed, large-scale RCTs are needed to further explore the effects of RTX on SDNS/FRNS.
In conclusion, RTX has been demonstrated to be efficient, well tolerated, and safe for children with FRNS/SDNS. Close monitoring is needed to help personalize RTX treatment in patients. Additional high-quality studies with larger sample sizes are needed to further identify any potential problems in effectiveness and safety.