Introduction
Cardiovascular diseases (CVDs) are the leading causes of morbidity and mortality worldwide [1, 2]. Atherosclerosis, the most common underlying cause of CVDs, is a chronic and progressive pathological condition characterized by lipid proliferation and inflammation in the artery walls [1, 2]. One of the main risk factors contributing to the early development of atherosclerosis is elevated levels of circulating low-density lipoprotein cholesterol (LDL-C) [3]. A major pathway of LDL-C clearance from the bloodstream is its uptake by hepatic LDL receptors (LDLR) [4]. The proprotein convertase subtilisin-like kexin type 9 (PCSK9) is a serine protease which bind to the LDLR and chaperones it for lysosomal degradation, thereby elevating the circulating levels of LDL-C [4]. Inhibiting PCSK9, the critical negative regulator of the LDLR, with monoclonal antibodies (mAbs) has been a milestone in lipid-lowering medication over the last decade and has gained increasing attention for preventing and managing atherosclerosis and CVDs [5–7]. On the other hand, long-term clinical usage of mAbs has drawbacks such as a short in vivo half-life, which necessitates frequent administration and high cost, specific tolerability issues, and the potential development of host anti-mAbs [8]. Active immunotherapy and vaccination techniques against PCSK9 have exploded in popularity to overcome these constraints [8]. Our group has recently designed a novel anti-PCSK9 vaccine formulation named Liposomal Immunogenic Fused PCSK9-Tetanus peptide plus Alum adjuvant (L-IFPTA+), which has shown fascinating results in different animal models [9–14]. The vaccine could significantly inhibit PCSK9 enzyme synthesis by promoting antibody production against it, followed by a considerable reduction in LDL-C levels in the blood of atherosclerotic mice [10].
MicroRNAs (miRNAs) are a class of small (~23 nucleotides) endogenous non-coding single-strand RNAs that regulate gene expression at the posttranscriptional level [15, 16]. They interact with the 3′ untranslated region (3′ UTR) of target mRNAs and lower protein synthesis by enhancing mRNA degradation, interfering with mRNA translation, or both [15, 17]. miRNAs play a pivotal role in the pathophysiology of the cardiovascular system [18]. They assist in regulating lipid metabolism through a complicated interactive mechanism involving gene regulatory networks and represent novel therapeutic target agents for human metabolic diseases [19, 20]. miR-27a has been shown to decrease LDLR levels by directly binding to its 3′-untranslated region (UTR) and indirectly by enhancing PCSK9, which improves LDLR degradation. miR-27a also directly downregulates the expression of LDLR-related protein 6 (LRP6) and LDLR-adapter protein 1 (LDLRAP1), key players in the LDLR pathway necessary for endocytosis of the LDLR-LDL-C complex in the liver by binding to their 3′-UTR [21]. On the other hand, miRNA-191 can directly interact with PCSK9 3′-UTR and regulate its expression [22]. Changes in MiR-30c appear to have an impact on circulating cholesterol and triglyceride levels [23]. This microRNA binds to the 3′-untranslated region of microsomal triglyceride transfer protein (MTP) mRNA and causes it to degrade, reducing MTP activity and apolipoprotein B (APOB) secretion and inhibiting VLDL synthesis [23]. MiR-30c also inhibits hepatic lipid synthesis by targeting the enzyme lysophosphatidyl glycerol acyltransferase 1 (LPGAT1) [23].
In the present study, we developed a non-nanoliposomal form of the L-IFPTA+ vaccine due to its more straightforward system and easier quality control, including PCSK9 immunogenic peptide fused tetanus peptide plus alum adjuvant. Here we investigated the effects of PCSK9 immunogenic peptide and alum adjuvant on the hepatic expression of miRNAs involved in the cholesterol homeostasis and PCSK9/LDLR pathway, including miRNA-27a, miRNA-30c, and miRNA-191 in normal immunized mice.
Material and methods
Vaccine preparation
We used the PCSK9 peptide epitope linked to a tetanus peptide epitope with the SIPWNLERITPVRkkAQYIKANSKFIGITEL sequence as the immunogen. The vaccine structure includes a short PCSK9 peptide as a B cell epitope inspired by the AFFiRiS group [9, 20] linked to a tetanus peptide as a T cell epitope, a pharmaceutically acceptable carrier [24] (Table I). The peptide was synthesized by ChinaPeptides Co, Ltd. (Shanghai, China), and the vaccine formulation was prepared by homogeneously mixing the PCSK9 immunogenic peptide with 0.4% alum adjuvant at a 1 : 1 ratio.
Table I
Sequences of the immunogenic peptides used in the present study. A 2-lysine-spacer sequence (kk) as the target sequence of cathepsin protease involved in antigen processing
| Peptide name | Sequence | Immunogenicity |
|---|---|---|
| PCSK9 | S-I-P-W-N-L-E-R-I-T-P-V-R | B cell epitope |
| Tetanus | A-Q-Y-I-K-A-N-S-K-F-I-G-I-T-E-L | T cell epitope |
| PCSK9 peptide vaccine | SIPWNLERITPVRkkAQYIKANSKFIGITEL |
Animals
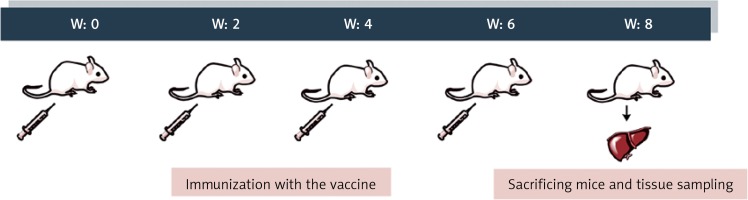
Animal studies were conducted on 6–8-week-old albino mice, prepared by Razi Vaccine and Serum Research Institute, Iran. All animal handling steps were commensurate with the animal welfare guidelines approved by the Organizational Ethics Committee and the Research Advisory Committee of Mashhad University of Medical Sciences (Mashhad, Iran). Environmental conditions were the same during all phases of the study. The animals were kept under standard temperature, humidity, and darkness/light cycle conditions and fed freely on a standard rodent diet and water ad-lib. One week after adaptation to the laboratory environment, 20 male albino mice were randomly divided into two vaccine and control groups (10 mice in each group).
Immunization and tissue sampling
Mice were immunized four times subcutaneously with 10 µg of peptide antigen at bi-weekly intervals. Mice in the control group received phosphate-buffered saline (PBS). Two weeks after the last immunization (W8), the animals were sacrificed following an intraperitoneal sodium thiopental 30 mg/kg injection (Figure 1). To assess the hepatic expression of microRNAs in vaccinated mice, mice’s livers were dissected, washed with saline, and stored in RNAlater Solution (Denazist, S-5062, Mashhad, Iran) immediately.
Hepatic RNA isolation and cDNA synthesis
To determine the hepatic expression levels of miRNAs, total RNA was extracted from 5–10 mg frozen hepatic tissues using BIOzol RNA lysis buffer (BN-0011.33, Bonyakhteh, Tehran, Iran) according to the manufacturer’s protocol with some modifications, such as increasing the incubation period and centrifugation to obtain the largest amount of miRNAs in the samples. The quantity and quality of the isolated RNA were evaluated using a Nanodrop2000 instrument (Thermo, Wilmington, DE, USA). About 5 µg of total RNA with absorbance of 1.8–2 at 260/280 nm was used for the initial polyadenylation step, followed by using the RT Stem-loop primer designed by Bonyakhteh company which was available in the BONmiR High Sensitivity MicroRNA 1st Strand cDNA Synthesis kit (BN-0011.17.2, Bonyakhteh, Tehran, Iran). The universal cDNA synthesis was completed via the thermocycler device for 10 min at 25°C, 60 min at 42°C, and 10 min at 70°C. The synthesized cDNA was stored at –20°C for future quantitative real-time PCR (qRT-PCR)
qRT-PCR
To assess the relative hepatic expression levels of miR-27a, miR-30c, and miR-191 the SYBR Green qPCR method was applied on the LightCycler 96 Instrument (Roche Diagnostics, Mannheim, Germany) using the BON microRNA QPCR master mix kit (BN-0011.17.4, Tehran, Iran) containing miRNA-specific primers (designed by Bonyakhteh company, Tehran, Iran) (Table II). All reactions were carried out in duplicate. The qPCR steps were performed according to the manufacturer’s instructions. After pre-incubation (95°C for 2 min), amplification was run for 40 cycles (95°C for 5 s and 60°C for 30 s). The miRNAs expression levels were measured using the Ct (cycle threshold) values and calculated using the comparative (2–ΔΔCt) method (fold change (FC)). U6 small nuclear RNA (U6snRNA) was used as an internal control to normalize miRNA expression.
Results
Hepatic miRNA expression levels in albino mice treated with the PCSK9 peptide vaccine
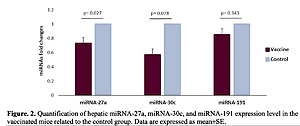
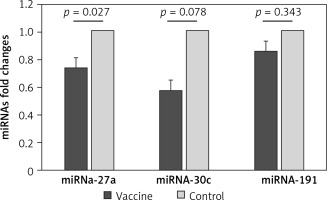
We evaluated the hepatic expression of miR-27a, miR-30c, and miR-191 in vaccinated mice compared to the control group and found that there was a significantly lower hepatic expression level of miR-27a in the vaccinated mice compared to the control mice (FC: 0.731 ±0.1, p = 0.027). Moreover, there was a borderline significantly lower hepatic expression level of miR-30c in the vaccinated mice compared to the control group (Fc: 0.569 ±0.1, p = 0.078). However, no significant difference was detected in the hepatic expression level of miR-191 between the vaccinated and control mice (FC: 0.852 ±0.1, p = 0.343) (Figure 2).
Discussion
Considering the critical importance of PCSK9 inhibition in dyslipidemia management, it is necessary to identify the underlying controlling mechanisms in which PCSK9 is involved. Moreover, discovering the mechanism of action of its inhibitors is highly desirable. miRNAs, as critical endogenous regulators, have a vital role in regulating cholesterol homeostasis involving genes including LDLR, PCSK9, and LDL-C. Therefore, to better understand the mechanism underlying PCSK9 inhibitors, including the PCSK9 peptide vaccine, it is important to detect whether the vaccine can affect miRNAs involved in cholesterol homeostasis. Here we observed a significant reduction in hepatic expression level of miRNA-27a after four vaccinations with the PCSK9 peptide vaccine.
miRNAs have been identified as players in the regulation of lipid homeostasis by a growing number of studies [25–30]. Several studies have demonstrated dysregulation of miRNA levels or miRNA targeted sites in CVD [16], underlining the relevant role of miRNAs in atherosclerosis [21, 31]. It has been reported that miR-27a dysregulation is linked to a wide range of diseases, including metabolic syndrome [32] nonalcoholic fatty liver disease (NAFLD) [33], diabetes [32, 34, 35] obesity [36] and gestational hypercholesterolemia [37]. Recent studies suggest that miRNA-27a may be a potential novel drug target in atherosclerosis and lipid metabolism [21, 33, 38]. In a study, Choi et al. reported that LDLR is downregulated by miRNA-27a, and during hepatic differentiation, LDLR levels increase as miRNA-27a expression decreases [38]. Also, a study by Liu et al. demonstrated that miRNA-27a was induced by oxLDL, so that inhibition of PCSK9 repressed this induction, suggesting that PCSK9 could reciprocally induce miRNA-27a [33]. Moreover, Alvarez et al. showed that overexpressing miRNA-27a in HepG2 cells led to a 40% decrease in LDLR levels directly through binding to its untranslated regions and indirectly through a 3-fold increase in PCSK9, which enhances LDLR degradation [21]. In addition, they indicated that miR-27a also directly inhibits other members of the LDLR pathway, particularly LRP6 and LDLRAP1, which are essential for endocytosis of the LDLR-LDL-C complex in the liver [21]. They also reported a 70% increase in the levels of LDLR and a 50% decrease in PCSK9 by inhibition of miRNA-27a using a specific LNA antisense oligonucleotide. Furthermore, they found a 50% decrease in miRNA-27a levels in HepG2 treated with Bay-11, an inhibitor of nuclear factor κB (NF-κB), indicating that NF-κB upregulates hepatic miRNA-27a, which may contribute to increasing LDL-C in NAFLD and atherosclerosis [21]. They also found that simvastatin, a well-known LDL-lowering drug, caused a dose-response increase in the miRNA-27a levels in HepG2 cells. Because miRNA-27a reduces LDLR levels while increasing PCSK9 levels, miRNA-27a upregulation might limit this drug’s effectiveness [21]. However, the PCSK9 peptide vaccine we developed, unlike simvastatin, could significantly reduce the hepatic expression of miRNA-27a in albino mice. This reduction of miRNA-27a can represent the anti-atherosclerotic and protective effects of this method of vaccination.
Thus, based on our findings, it is likely that the PCSK9 peptide vaccine helps manage LDL-C and atherosclerosis through the metabolic pathways in which miRNA-27a is involved, particularly the LDLR pathway [38–42]. Nevertheless, it may not work through pathways in which miRNA-30c and miRNA-191 are involved, including the pathway that reduces MTP activity and APOB secretion, leading to VLDL synthesis inhibition, regulated by miRNA-30c, and the pathway which leads to PCSK9 degradation regulated by miRNA-191, due to their non-significant differences between the vaccine and control groups. Since PCSK9 has also been proposed to be involved in pathways beyond LDL control [43–46], it would be interesting to explore if the altered levels of microRNAs mediate these pleiotropic actions.
To sum up, the results presented here provide an insight into the underlying mechanisms of the PCSK9 peptide vaccine and further evidence supporting the potential of the PCSK9 peptide vaccine as a therapeutic method of vaccination against atherosclerosis.