Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
NEONATOLOGY / CLINICAL RESEARCH
Unraveling the impact of neonatal jaundice on allergic diseases: a Mendelian randomization study
1
Department of Pediatrics, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang City, China
Submission date: 2024-08-17
Final revision date: 2025-02-02
Acceptance date: 2025-02-17
Online publication date: 2025-04-27
Corresponding author
Jian Wu
Department of Pediatrics The First Affiliated Hospital Jiangxi Medical College Nanchang University No. 17 Yongwaizheng St Donghu District Nanchang City Jiangxi Province 330000, China
Department of Pediatrics The First Affiliated Hospital Jiangxi Medical College Nanchang University No. 17 Yongwaizheng St Donghu District Nanchang City Jiangxi Province 330000, China
Xiao Chen
Department of Pediatrics The First Affiliated Hospital Jiangxi Medical College Nanchang University No. 17 Yongwaizheng St Donghu District Nanchang City Jiangxi Province 330000, China
Department of Pediatrics The First Affiliated Hospital Jiangxi Medical College Nanchang University No. 17 Yongwaizheng St Donghu District Nanchang City Jiangxi Province 330000, China
Gaole Yuan
Department of Pediatrics The First Affiliated Hospital Jiangxi Medical College Nanchang University No. 17 Yongwaizheng St Donghu District Nanchang City Jiangxi Province 330000, China
Department of Pediatrics The First Affiliated Hospital Jiangxi Medical College Nanchang University No. 17 Yongwaizheng St Donghu District Nanchang City Jiangxi Province 330000, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Neonatal jaundice, a condition characterized by elevated bilirubin levels in newborns, is prevalent, affecting up to 60% of term infants. Previous observational studies have linked neonatal jaundice to an enhanced risk of allergic diseases such as asthma, atopic dermatitis (AD), allergic conjunctivitis (AC), allergic rhinitis (AR), and urticaria. However, the causal relationship remains unclear due to potential confounding factors and reverse causality.
Material and methods:
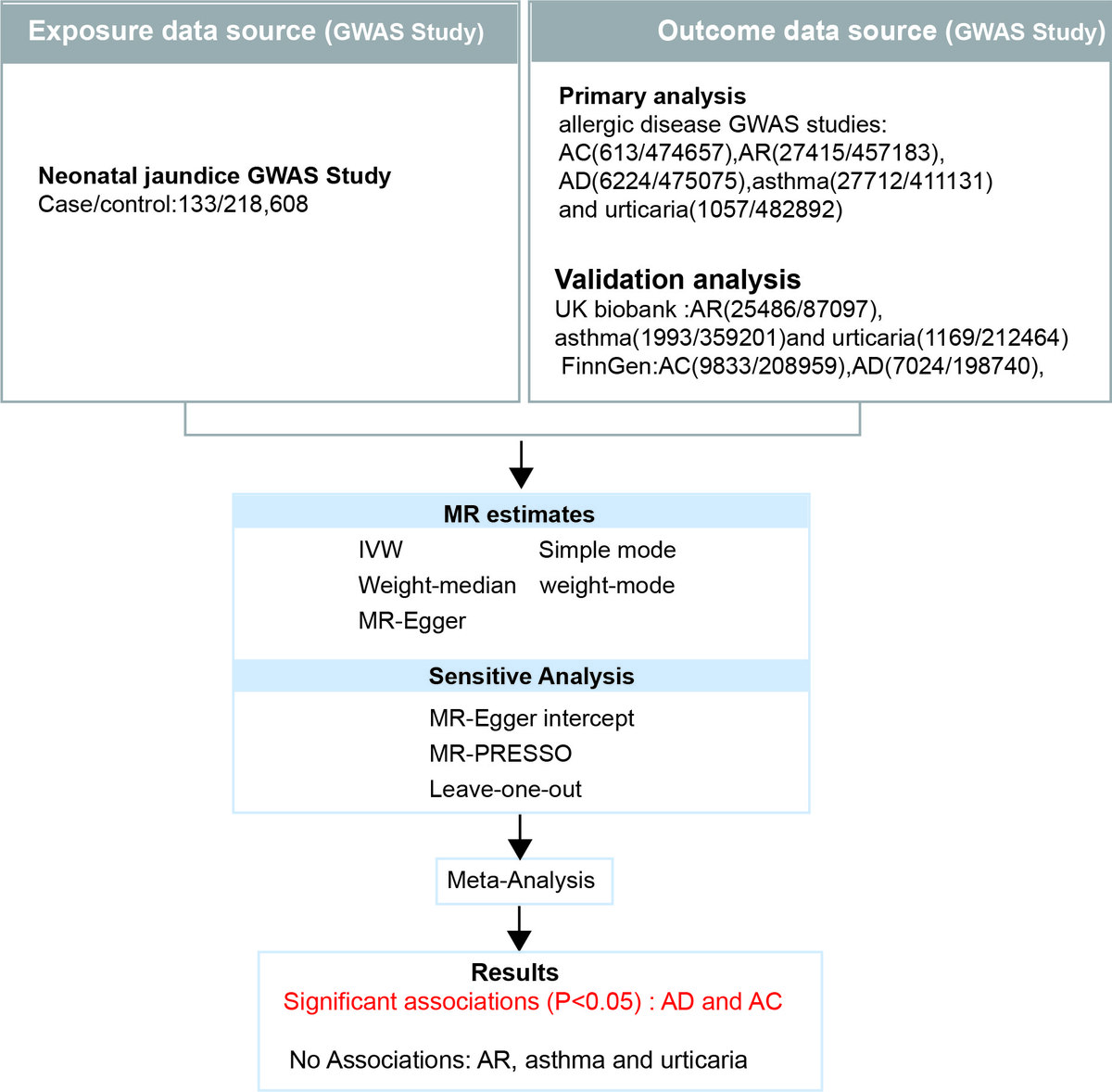
We conducted a two-sample MR analysis using genetic variants as instrumental variables. Data from large-scale GWAS in European populations were used, including exposure data for neonatal jaundice and outcome data for five common allergic diseases. MR analysis was performed using the inverse variance weighted (IVW) method, with additional sensitivity analyses conducted using MR-Egger regression, weighted median, simple mode, and weighted mode methods.
Results:
MR analysis revealed a significant causal association between neonatal jaundice and an increased risk of AD (OR = 1.0141, 95% CI: 1.0041–1.0241, p = 0.006) and AC (OR = 1.0119, 95% CI: 1.0014–1.0226, p = 0.026). No significant association was found between neonatal jaundice and pediatric asthma, urticaria, or AR. Sensitivity analyses indicated no evidence of pleiotropy, and no individual SNPs substantially influenced the results, confirming the robustness of our findings.
Conclusions:
This study provides evidence for a causal association between neonatal jaundice and an increased risk of AD and AC. These findings suggest that neonatal jaundice may be a modifiable risk factor for AD and AC, highlighting the importance of neonatal jaundice management and further research on potential preventive strategies.
Neonatal jaundice, a condition characterized by elevated bilirubin levels in newborns, is prevalent, affecting up to 60% of term infants. Previous observational studies have linked neonatal jaundice to an enhanced risk of allergic diseases such as asthma, atopic dermatitis (AD), allergic conjunctivitis (AC), allergic rhinitis (AR), and urticaria. However, the causal relationship remains unclear due to potential confounding factors and reverse causality.
Material and methods:
We conducted a two-sample MR analysis using genetic variants as instrumental variables. Data from large-scale GWAS in European populations were used, including exposure data for neonatal jaundice and outcome data for five common allergic diseases. MR analysis was performed using the inverse variance weighted (IVW) method, with additional sensitivity analyses conducted using MR-Egger regression, weighted median, simple mode, and weighted mode methods.
Results:
MR analysis revealed a significant causal association between neonatal jaundice and an increased risk of AD (OR = 1.0141, 95% CI: 1.0041–1.0241, p = 0.006) and AC (OR = 1.0119, 95% CI: 1.0014–1.0226, p = 0.026). No significant association was found between neonatal jaundice and pediatric asthma, urticaria, or AR. Sensitivity analyses indicated no evidence of pleiotropy, and no individual SNPs substantially influenced the results, confirming the robustness of our findings.
Conclusions:
This study provides evidence for a causal association between neonatal jaundice and an increased risk of AD and AC. These findings suggest that neonatal jaundice may be a modifiable risk factor for AD and AC, highlighting the importance of neonatal jaundice management and further research on potential preventive strategies.
REFERENCES (47)
1.
Shin YH, Hwang J, Kwon R, et al. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023; 78: 2232-54.
2.
Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol 2010; 125: S81-94.
3.
Holloway JW, Prescott SL. The Origins of Allergic Disease. In: Middleton’s Allergy Essentials. O’Hehir RE, Holgate ST, Sheikh A, eds. Elsevier 2017; 29-50.
4.
Krempski JW, Dant C, Nadeau KC. The origins of allergy from a systems approach. Ann Allergy Asthma Immunol 2020; 125: 507-16.
5.
Wei CC, Lin CL, Shen TC, Kao CH. Neonatal jaundice and risks of childhood allergic diseases: a population-based cohort study. Pediatr Res 2015; 78: 223-30.
6.
Tanimizu N. The neonatal liver: normal development and response to injury and disease. Semin Fetal Neonatal Med 2022; 27: 101229.
7.
Kuniyoshi Y, Tsujimoto Y, Banno M, Taito S, Ariie T. Neonatal jaundice, phototherapy and childhood allergic diseases: an updated systematic review and meta-analysis. Pediatr Allergy Immunol 2021; 32: 690-701.
8.
Egeberg A, Andersen YM, Gislason G, Skov L, Thyssen JP. Neonatal risk factors of atopic dermatitis in Denmark – results from a nationwide register-based study. Pediatr Allergy Immunol 2016; 27: 368-74.
9.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. In: Livingston EH, Lewis RJ, eds. JAMA Guide to Statistics and Methods. New York, NY: McGraw-Hill Education 2019.
10.
Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nat Rev Methods Primers 2022; 2: 6.
11.
Li L, Wei L, Liu K, Dong A, Gong Y. Association between the IGF family members and UTUC: a Mendelian randomization study. Arch Med Sci 2024. DOI: https://doi.org/10.5114/aoms/1....
12.
Wang J, Sun Z, Zhong Y, et al. Sleep disturbances and heart failure: observational study and Mendelian randomization study. Arch Med Sci 2025; 21: 1222-32.
13.
Ding L, Chen Q, Liang H, Shen M, Zheng M, Li Z. Physical activities and breast cancer: a Mendelian randomization study. Arch Med Sci 2024; 20: 1957-67.
14.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018; 362: k601.
15.
Zhu Z, Zheng Z, Zhang F, et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun 2018; 9: 224.
16.
Zeng P, Zhao Y, Qian C, et al. Statistical analysis for genome-wide association study. J Biomed Res 2015; 29: 285-97.
17.
Zhang YC, Fan KY, Wang Q, et al. Genetically determined levels of mTOR-dependent circulating proteins and risk of multiple sclerosis. Neurol Ther 2023; 12: 751-62.
18.
Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Human Genet 2007; 81: 559-75.
19.
Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263-5.
20.
Wang Y, Zhao Z, Wang R, Hu X. Genetic links between gastrointestinal disorders and kidney stone disease: insights from genome-wide cross-trait analysis. Kidney360. 2025; 6: 616-26.
21.
Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018; 7: e34408.
22.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014; 23: R89-98.
23.
Emmert DB, Stoehr PJ, Stoesser G, Cameron GN. The European Bioinformatics Institute (EBI) databases. Nucleic Acids Res 1994; 22: 3445-9.
24.
Bowden J, Hemani G, Davey Smith G. Invited Commentary: Detecting individual and global horizontal pleiotropy in Mendelian randomization-a job for the humble heterogeneity statistic? Am J Epidemiol 2018; 187: 2681-5.
25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018; 50: 693-8.
26.
Das RR, Naik SS. Neonatal hyperbilirubinemia and childhood allergic diseases: a systematic review. Pediatr Allergy Immunol 2015; 26: 2-11.
27.
Apfelbacher CJ, Diepgen TL, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy 2011; 66: 206-13.
28.
Huang L, Bao Y, Xu Z, et al. Neonatal bilirubin levels and childhood asthma in the US Collaborative Perinatal Project, 1959-1965. Am J Epidemiol 2013; 178: 1691-7.
29.
Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol 2010; 21: e733-9.
30.
Ku MS, Sun HL, Sheu JN, Lee HS, Yang SF, Lue KH. Neonatal jaundice is a risk factor for childhood asthma: a retrospective cohort study. Pediatr Allergy Immunol 2012; 23: 623-8.
31.
Aspberg S, Dahlquist G, Kahan T, Källén B. Is neonatal phototherapy associated with an increased risk for hospitalized childhood bronchial asthma? Pediatr Allergy Immunol 2007; 18: 313-9.
32.
Sun HL, Lue KH, Ku MS. Neonatal jaundice is a risk factor for childhood allergic rhinitis: a retrospective cohort study. Am J Rhinol Allergy 2013; 27: 192-6.
33.
Singh M, Ranjan Das R. Probiotics for allergic respiratory diseases: putting it into perspective. Pediatr Allergy Immunol 2010; 21: e368-76.
34.
Liu Y, Li P, Lu J, et al. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol 2008; 181: 1887-97.
35.
Procianoy RS, Silveira RC, Fonseca LT, Heidemann LA, Neto EC. The influence of phototherapy on serum cytokine concentrations in newborn infants. Am J Perinatol 2010; 27: 375-9.
36.
Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 2004; 113: 1776-82.
37.
Dani C, Masini E, Bertini G, et al. Role of heme oxygenase and bilirubin in oxidative stress in preterm infants. Pediatr Res 2004; 56: 873-7.
38.
Nag N, Halder S, Chaudhuri R, Adhikary S, Mazumder S. Role of bilirubin as antioxidant in neonatal jaundice and effect of ethanolic extract of sweet lime peel on experimentally induced jaundice in rat. Indian J Biochem Biophys 2009; 46: 73-8.
39.
Kurt A, Aygun AD, Kurt AN, Godekmerdan A, Akarsu S, Yilmaz E. Use of phototherapy for neonatal hyperbilirubinemia affects cytokine production and lymphocyte subsets. Neonatology 2009; 95: 262-6.
40.
Bahraini P, Karami M, Sabzehei M, Eslamian M. Jaundiced neonates receiving phototherapy and risk of atopic dermatitis in the first 2 years of life: a case-control study. J Pediatr Perspect 2019; 7: 10397-403.
41.
Vítek L, Kotal P, Jirsa M, et al. Intestinal colonization leading to fecal urobilinoid excretion may play a role in the pathogenesis of neonatal jaundice. J Pediatr Gastroenterol Nutr 2000; 30: 294-8.
42.
Zhang K, Fan S, Lv A, Ma Y, Fang X, Zhang J. Integrated analysis of microbiota with bile acids for the phototherapy treatment of neonatal jaundice. Arch Med Sci 2023; 19: 401-10.
43.
Xue Y, Zhang L, Chen Y, Wang H, Xie J. Gut microbiota and atopic dermatitis: a two-sample Mendelian randomization study. Front Med 2023; 10: 1174331.
44.
Moniaga CS, Tominaga M, Takamori K. An altered skin and gut microbiota are involved in the modulation of itch in atopic dermatitis. Cells 2022; 11: 3930.
45.
He JH, Zhao XG, Sun F, Peng WQ, Li HY, Li H. Clinical study on prevention of atopic dermatitis by oral administration of probiotics in infants. Arch Med Sci 2023; 19: 101-6.
46.
Abdel Ghany EA, Hussain NF, Botros SK. Glutathione S-transferase gene polymorphisms in neonatal hyperbilirubinemia. J Investig Med 2012; 60: 18-22.
47.
Dasari S, Gonuguntla S, Ganjayi MS, Bukke S, Sreenivasulu B, Meriga B. Genetic polymorphism of glutathione S-transferases: relevance to neurological disorders. Pathophysiology 2018; 25: 285-92.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.