Introduction
In the United States, the prevalence of obesity (body mass index (BMI) ≥ 30 kg/m2) is 42.4%, and the prevalence of severe obesity (BMI ≥ 40) is 9.2% [1]. The public health burden of obesity is only expected to increase, as nearly half of the U.S. adult population is expected to be classified as obese and nearly a quarter of the U.S. adult population is expected to be severely obese by 2030 [2]. Obesity increases patient risk of developing certain cancers, including breast cancer, due to multifactorial mechanisms, including hormonal sensitive pathways driven by excess aromatase-mediated peripheral estrogen production [3]. Further, obesity is associated with shorter overall longevity and a greater proportion of life years with cardiovascular disease (CVD) and associated morbidity [4]. At present, the leading cause of all-cause mortality in the United States is heart disease (23%), followed closely by cancer (21%) [5]. Despite clear deleterious health implications, obesity has been linked to improved outcomes in certain cancer patients, although the implications remain a significant point of community discussion [6, 7].
In 2022, it was estimated that 1,918,030 new cases of cancer would be diagnosed in the United States, with breast cancer and lymphoma comprising 290,560 and 89,010 new cases, respectively [5]. While 609,360 people were expected to die from cancer in 2022, there have been continuous decreases in the risk of death from cancer since 1991 [5]. In conjunction with the decreased risk of death, there exist substantially more cancer survivors. It was estimated that, as of 2019, there were 16.9 million people with a history of cancer, which is expected to grow to exceed 22.1 million by 2030 [8].
Anthracyclines (AC) are cytostatic antineoplastic agents that were first discovered in the 1950s. Within the anthracycline drug class, doxorubicin (Adriamycin) and daunorubicin are the two most commonly used agents. Mechanistically, AC interact with topoisomerase II, contributing to mitochondrial toxicity and triggering cellular apoptosis. These agents are mainstays of care in leukemia, lymphoma, breast, stomach, uterine, ovarian, bladder, and lung cancers [9].
Trastuzumab (TRA) is an anti-HER2-neu monoclonal antibody that is a mainstay of chemotherapy in human epidermal growth factor receptor 2 positive (HER2+) cancers, most commonly in breast cancer [10]. Approximately 25–30% of patients with breast cancer have the subtype characterized by HER2/neu gene amplification, which is associated with more aggressive disease [11]. Trastuzumab is a key component of all recommended chemotherapy regimens for HER2+ breast cancer for both neoadjuvant and adjuvant therapy with substantial clinical benefit.
While both AC and TRA are highly efficacious chemotherapeutic agents, they may precipitate cardiotoxicity, which may necessitate changes in chemotherapy dosage, schedule, and regimen. Chemotherapy-induced cardiotoxicity is an important clinical phenomenon that can manifest as heart failure, cardiac dysrhythmias, or myocardial ischemia. It is a particularly significant impairing side effect of AC and HER2-targeted therapy, identified commonly as abnormal left ventricular ejection fraction (LVEF) and symptoms of congestive heart failure. The incidence of cardiotoxicity ranges from 3% to 7% with TRA monotherapy and is 27% with concomitant AC and TRA [12]. Clinicians following these patients must remain vigilant throughout the patient’s treatment course given the high incidence of cardiotoxicity. A multi-disciplinary approach, determined by the severity of cardiotoxicity, must be pursued to determine potential mitigatory strategies and the role for continued or alternative therapy. Further, chemotherapy-induced cardiotoxicity is associated with long-term impairment and may increase the future risk of developing CVD [13]. As the number of cancer survivors continues to increase, it is of the upmost importance to identify risk factors and effective treatments to curtail the impact of chemotherapy-associated cardiotoxicity.
There are known risk factors for the development of cardiotoxicity, and these remain of significant interest for cardiologists and oncologists alike. Given the prevalence of obesity in the cancer and CVD population, it is important to evaluate its relationship within the treatment paradigm for these disease states. Interestingly, obesity significantly increases the half-life of doxorubicin; if a linear relationship to weight and cardiotoxicity was identified, it could be proposed for future study to dose AC based on lean body mass to decrease the risk of toxicity [14]. Further, as doses of AC are calculated based on body surface area (BSA), and doses of TRA are calculated based on body weight, an obese patient would receive a higher absolute dose of the chemotherapy or targeted therapy, potentially increasing the risk of cardiotoxic side effects.
Our study aimed to investigate the relationship between obesity and the development of cardiotoxicity following anthracycline- or TRA-based chemotherapy.
Material and methods
Study aim, design, and setting
This was a single-center study at a large community hospital in Northeastern Pennsylvania. We conducted a retrospective cohort study of breast cancer and lymphoma patients who received chemotherapy with AC or TRA over a 5-year period from January 1, 2008, to December 31, 2012. The primary objective was to assess the incidence of cardiotoxicity in patients who received AC or TRA and evaluate the association between obesity (defined as BMI ≥ 30) and the likelihood of developing cardiotoxicity. We hypothesized that patients taking AC and/or TRA with a BMI greater than 30 would have greater odds of cardiotoxicity following chemotherapy compared to patients with a BMI of 30 or lower. Cardiotoxicity in this study was defined as LVEF less than 50% on multiple gated acquisition scans (MUGA) or echocardiogram (ECHO) or evidence of ischemia or infarction on ECHO or cardiac catheterization. A total of 368 patients treated with AC or TRA met our inclusion criteria. Patients were excluded if they were less than 18 years of age, pregnant, or had preexisting heart failure or severe cardiomyopathy with an EF of less than 30%. Sixteen patients met criteria for cardiotoxicity in this sample.
Participant characteristics
Baseline characteristics were collected, including cancer type; chemotherapy type, dose, and duration; age, gender, height, weight, BMI, and relevant past cardiac history. Hospital and outpatient evaluations were assessed for evidence of congestive heart failure, and the results of echocardiogram, EKG, MUGA, BNP, NT-proBNP, troponin, stress testing, and cardiac catheterizations were assessed if available. All patients underwent baseline cardiac function evaluation with either echocardiogram or MUGA. The role for repeat functional assessments was determined and performed according to the standard adult protocol. The dosing of anthracycline therapy varied by cancer type: 60 mg/m2 for breast cancer and 50 mg/m2 for lymphoma. However, the cumulative dose of anthracycline therapy received was calculated in each patient to determine whether there was an absolute dose value that predicted cardio-toxicity. All data were collected from the electronic medical record (EMR), including MOSAIQ, EPIC, and Centricity (CE). Ethical approval was given by the institutional review board.
Statistical analysis
Descriptive statistics were generated for the entire sample. Categorical variables were presented as frequencies and percentages. Means and standard deviations were generated for all continuous variables found to be approximately normally distributed; otherwise, the median and interquartile range were used.
Bivariate analyses were conducted to determine the association between demographic and clinical variables with cardiotoxicity status. The χ2 test or Fisher’s exact test was used for categorical variables, and the independent samples t-test or Mann Whitney-U test was used for continuous variables. A simple logistic regression model was generated to further examine the association between BMI and developing cardiotoxicity. Two models were run: the first used BMI as a continuous predictor and the second used BMI as a dichotomous variable based upon the cut-point for obesity (> 30 and ≤ 30 kg/m2).
To assess whether BMI was independently associated with cardiotoxicity, two multivariate logistic regression models were generated. The first model used BMI as a continuous predictor, and the second used BMI as a dichotomous variable based on the cut-point for obesity (> 30 and ≤ 30 kg/m2). In a stepwise fashion, any variables found to be moderately associated with cardiotoxicity upon bivariate analysis (p < 0.20) that also met the assumptions for logistic regression were included in the final model. All model assumptions, including multicollinearity, were assessed. The final models controlled for age, chemotherapy type, hypertension, and past heart problems of coronary artery disease (CAD) and arrhythmia.
All analyses were conducted using SAS version 9.4 and were two-tailed with a p-value of less than 0.05 considered statistically significant. The data analysis for this paper was generated using SAS software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 368 patients were included in the sample, 16 of whom developed cardiotoxicity. Patients were demographically similar as age, gender, and race had similar distributions between the two groups. In the entire sample, the average age was 54 years, 79.4% of patients were female and 76.9% of patients were of white race. The median BMI in the entire sample was 28.3 kg/m2. Most patients (58.4%) were not classified as obese. The incidence of cardiotoxicity in this sample during the designated study period was 4.35% (16 out of 368 total patients). Of the patients on AC only (N = 240) the incidence was 5%, on TRA only (N = 97) the incidence was 3.1%, and on both AC and TRA (N = 31) the incidence was 3.2% (Table I).
Table I
Comparison of patient demographics in the entire sample and by cardiotoxicity status
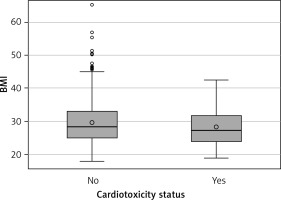
In the bivariate analysis of obesity with cardiotoxicity, no significant association was found. 41.8% of patients without cardiotoxicity were obese, and 37.5% of patients with cardiotoxicity were obese. On a continuous scale, there was no significant difference in median BMI between patients without cardiotoxicity (28.3, IQR: 24.9–32.9) and those with cardiotoxicity (27.2, IQR: 24.0–31.7) (Figure 1).
Figure 1
Body mass index (BMI) by cardiotoxicity status (n = 368). No cardiotoxicity (n = 252), Cardiotoxicity (n = 16)
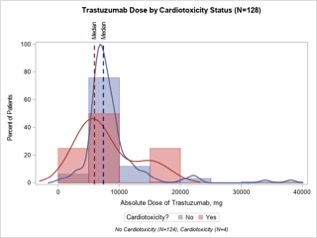
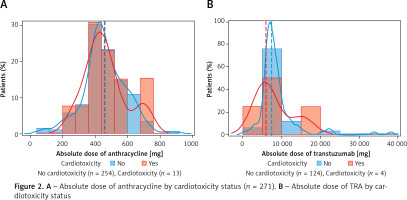
In the assessment of patient comorbidities and medical history, the presence of previous heart problems was significantly associated with cardiotoxicity status. The previous heart problems of CAD, arrhythmia, and functional cardiomyopathy were significantly associated with cardiotoxicity (p = 0.0150, p = 0.0011, p < 0.0001, respectively). No other significant associations were found between groups. Compared to patients without cardiotoxicity, a greater proportion of patients had hypertension (56.3% vs. 33.2%), were on anthracycline only (75.0% vs. 64.8%) and had a higher average anthracycline dose (461.9 ±128.3 mg vs. 457.2 ±130.5 mg) (Table II, Figure 2 A). Figures 2 A and B plot the absolute dose of anthracycline and TRA stratified by cardiotoxicity status. A greater proportion of patients with cardiotoxicity had a higher absolute dose of AC, whereas a greater proportion of patients without cardiotoxicity had a higher absolute dose of TRA.
Table II
Comparison of patient comorbidities and medical history in the entire sample and by cardiotoxicity status
Figure 2
A – Absolute dose of anthracycline by cardiotoxicity status (n = 271). B – Absolute dose of TRA by cardiotoxicity status
In the simple logistic regression models, for every one-unit increase in BMI, the odds of cardiotoxicity were 0.969 (95% CI: 0.895–1.049), indicating a slight protective effect of BMI on cardiotoxicity. This was also seen when looking at BMI dichotomized into obesity categories: the odds of cardiotoxicity in obese patients was lower compared to non-obese patients (0.837, 95% CI: 0.298–2.353) (Table III).
Table III
Odds ratios for the effect of BMI (kg/m2) and obesity on cardiotoxicity
| Parameter | BMI [kg/m2] (n = 368) | Obesity (n = 368) |
|---|---|---|
| Odds ratio (95% CI) | 0.969 (0.895–1.049) | 0.837 (0.298–2.353) |
In the multivariate model using BMI as a continuous predictor, the only significant predictor of cardiotoxicity was a previous history of arrhythmia (Table IV). In patients with a previous history of arrhythmia, the odds of cardiotoxicity were 5.01 (95% CI: 1.47–17.10, p = 0.0102) compared to patients without a history of arrhythmia, controlling for BMI, age, hypertension, chemotherapy type, and CAD. Similar to the results from the simple logistic regression model for BMI, an increase in BMI was associated with decreased odds of developing cardiotoxicity when controlling for all other variables (OR = 0.957, 95% CI: 0.88–1.04). The same effect was seen with age; for every 1-year increase in age, the adjusted odds of cardiotoxicity were 0.984 (95% CI: 0.94–1.03). Patients on AC compared to TRA had greater odds of cardiotoxicity (OR = 1.38, 95% CI: 0.36–5.23). Likewise, patients with hypertension and CAD also had nearly double the odds of cardiotoxicity compared to patients without the conditions (OR = 1.98 and 2.62, respectively). In the adjusted model using obesity, nearly identical results were seen for all covariates. Obesity was protective when adjusting for all other variables (OR = 0.704, 95% CI: 0.24–2.10) (Table V).
Table IV
Odds ratios for the effect of BMI (kg/m2) on cardiotoxicity controlling for age, chemotherapy type, hypertension, CAD, and arrhythmia
Table V
Odds ratios for the effect of obesity on cardiotoxicity controlling for age, chemotherapy type, hypertension, CAD, and arrhythmia
Discussion
Anthracycline-induced cardiotoxicity
Anthracycline-induced cardiotoxicity can occur along a spectrum of time points. Acute cardiotoxicity is a rare manifestation of toxicity, and typically occurs in the first 2 weeks after initial infusion [15]. Most commonly, early-onset toxicity will occur within the first year of treatment as an asymptomatic decline in LVEF that may progress to heart failure. Late-onset toxicity does occur and can present up to a decade after the initial treatment [15]. Importantly, anthracycline-induced cardiotoxicity is cumulative and dose-dependent, with studies showing that cumulative dose thresholds exceeding 300 mg/m2 were associated with declines in left ventricular ejection fraction [9]. Though not statistically significant, the patients who experienced cardiotoxicity in our cohort had a higher average dose of AC.
Risk factors associated with an increased incidence of anthracycline-induced cardiotoxicity include extremes of age, female gender, prior mediastinal radiation therapy, hypertension, cardiac disease, and weight ≥ 70 kg or BMI ≥ 27 kg/m2 [9, 16, 17]. Concurrent TRA and anthracycline use further increases the risk of developing heart failure or cardiomyopathy [18]. Our study, while a single-center study with a smaller patient population than that of Guenancia et al., did not identify an association between obesity and cardiotoxicity. We did find statistically significant associations between cardiotoxicity and pre-existing coronary artery disease, arrhythmia, and functional cardiomyopathy. After controlling for age, chemotherapy type, hypertension, CAD, and BMI, we found that pre-existing arrhythmia was independently associated with a five-fold increase in risk for cardiotoxicity. When adjusting for all these variables, obesity was found to be protective against cardiotoxicity. This finding is not surprising, as arrhythmias, and particularly atrial fibrillation, have been associated with increased mortality risk and progression of heart failure [19]. It has also been well documented in the literature that patients with pre-existing CVD have an increased risk of chemotherapy-associated cardiotoxicity [20]. It is possible that patients with pre-existing arrhythmia have an underlying condition that increases their risk for cardiotoxic complications from chemotherapy.
Over time, research has shown several cardioprotective strategies for chemotherapy regimens including anthracycline. Dexrazoxane, an iron chelator that reduces superoxide free radical formation, has been shown to reduce the incidence of heart failure and sub-clinical declines in LVEF in patients receiving AC [21, 22]. Additionally, continuous infusions and liposomal formulations of AC reduce the risk of cardiotoxicity [15, 22]. There is conflicting data about the utility of neurohormonal antagonists (i.e. aldosterone receptor antagonists and ACE inhibitors) and β-blockers in preventing anthracycline-induced cardiotoxicity, and expert consensus recommendations vary [15, 22]. While one small study found clinical benefit for spironolactone, a different study examining the use of eplerenone was discontinued early for futility [22]. In a meta-analysis of patients receiving AC with and without TRA, ACE-inhibitors did not reduce the risk of development of cardiotoxicity, whereas β-blockers attenuated declines in LVEF and significantly reduced new heart failure diagnoses [23]. In trials examining prophylactic combination therapy with ACE-Is or ARBs with β-blockers, results have been inconsistent, with some trials showing benefit and others failing to show benefit [22]. The National Comprehensive Cancer Network Survivorship guidelines mention that β-blockers and angiotensin-converting enzyme inhibitors may be utilized to prevent cardiac remodeling during therapy; however, this should only be done after thorough evaluation of the patient with balancing of risks and benefits [24]. For patients who develop anthracycline-induced heart failure, standard heart failure medical management is preferred, with goal-directed medical therapy and symptomatic management [9].
Trastuzumab-induced cardiotoxicity
TRA-induced cardiotoxicity differs from anthracycline-induced cardiotoxicity in that it is not an irreversible dose-dependent toxicity, but rather occurs due to reversible myocyte dysfunction [25]. Among TRA-treated patients aged 66 and older who developed CHF, 68.8% of cases occurred within the first 12 months after treatment initiation [26]. Once a week administration is associated with an increased risk [26]. The majority of patients who experience TRA-induced cardiotoxicity experience cardiac recovery upon cessation of TRA therapy [25–27]. TRA-induced cardiotoxicity is managed slightly differently than anthracycline-induced cardiotoxicity, as cessation of TRA will generally lead to recovery in cardiac function. There is some evidence to suggest that prophylactic initiation of β-adrenergic antagonists and angiotensin converting enzyme inhibitors may prevent TRA-induced cardiotoxicity [23, 28].
Well-established cardiac risk factors such as age, hypertension, diabetes, and CAD are generally associated with an increased risk of TRA-induced cardiotoxicity [22, 25]. Among patients aged 66 and older, patients treated with TRA were more likely to develop CHF or cardiomyopathy [18]. Risk factors for heart failure or cardiomyopathy were older age, hypertension, comorbidities (coronary artery disease, cerebrovascular disease, diabetes, renal failure, and arrhythmia), and previous or concurrent anthracycline exposure [29]. Concurrent use of AC and TRA has been shown to increase the risk of developing heart failure or cardiomyopathy, regardless of age [30]. Among patients older than 80 who were treated with TRA, coronary artery disease and hypertension were associated with an increased risk [26]. A systematic review found that the combination of patients with breast cancer who were either overweight (BMI 25 to 29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) was at a significantly greater risk of developing cardiotoxicity after AC and a sequential anthracycline and TRA regimen; however, this was without controlling for associated cardiac risk factors [31]. In our cohort, there was no statistically significant difference between treatment type (AC, TRA, or both) and cardiotoxicity. Prior chest radiation, age, race, and sex, while all commonly described risk factors in the literature, were not significant predictors of cardiotoxicity in our cohort.
Obesity and cardiotoxicity
The reported incidence of cardiotoxicity for patients receiving cancer chemotherapy varies according to how it is defined, with clinical decompensation occurring in 2% to 4%, subclinical structural change in 9% to 11%, and a biomarker rise in 30 to 35% of cancer chemotherapy patients [32]. In our study, the incidence of cardiotoxicity was 4.3%. The cardiac side effects of AC and TRA are evaluated by a decrease in LVEF as per the European Society of Cardiology guidelines [33]. We followed the same cutoff for cancer treatment and cardiovascular toxicity defined as a decrease of LVEF of at least 10 percentage points to an LVEF less than 50%. The relatively low incidence of cardiotoxicity in our study can be explained by a younger population and low risk factor profile [34].
Our study showed that obese patients had a lower incidence of cardiotoxicity (3.9%) than their non-obese counterparts (4.6%). Also, the odds of developing cardiotoxicity with AC and TRA did not increase with an increase in the BMI, suggesting that obesity could have a benign or even a slightly protective effect on cardiotoxicity development. However, these observed differences were not statistically significant. While several studies have provided evidence that obese patients with breast cancer experience worse outcomes than their non-obese counterparts, prior studies on the effect of obesity on cardiotoxicity in patients receiving chemotherapy have shown conflicting results [35, 36]. The French Multicenter CANTO (Cancer Toxicities) study aimed to examine the association of BMI and cardiotoxicity in patients with breast cancer treated with AC and/or TRA. They found obesity to be independently associated with an increased risk of cardiotoxicity [37]. A recent meta-analysis on 7,488 patients showed that obese patients are at an increased risk for AC-induced cardiotoxicity [38]. The investigators postulated that an increased risk of AC-induced cardiotoxicity was observed in obese patients due to increased oxidative stress and metabolic changes such as increased leptin and decreased adiponectin levels [39]. However, these studies were limited as they did not control for associated cardiac risk factors or lifestyle factors that may influence survival and did not perform a meta-regression to control for other factors, as performed in our study.
In a pooled analysis of two randomized controlled trials, obesity showed no impact on breast cancer prognosis with anthracycline adjuvant chemotherapy [40]. Similarly, in our study, we did not find any overall increased risk of obesity leading to worsening cardiac function in patients receiving chemotherapy. One of the limitations of our study is the small cohort of patients developing cardiotoxicity, which limits the generalization of our results. However, our results are hypothesis-generating and show that obese patients without other risk factors are not at higher risk of developing cardiotoxicity from the chemotherapeutic agents. Also, our results show that normal or underweight patients would benefit from careful cardiac screening and close follow-up during and after chemotherapy.
Even though obesity is generally expected to place patients at a higher risk of developing cardiovascular diseases, some studies have shown an inverse relationship between obesity and the prognosis of established cardiovascular disease [41–43]. This phenomenon, termed the ‘obesity paradox’, has been mainly observed in the heart failure patient population. It is postulated that as patients with heart failure enter a hypercatabolic state with associated hypoalbuminemia and cardiac cachexia, obese patients have higher metabolic reserves, and therefore, a more favorable prognosis. While the obesity paradox has not been previously described in patients receiving chemotherapy, chemotherapy in cancer patients results in a similarly increased catabolic state. Obesity and maintenance of body weight during the initial period of chemotherapy can be potentially independent predictors of survival in cancer patients [44]. Some studies have shown that survival outcomes follow an approximately U-shaped pattern [45]. However, the comparison between obese and morbidly obese patients was not possible in our study due to the low number of subjects in the cardiotoxicity group.
Most of the data obtained for the association between obesity and cardiotoxicity in patients receiving chemotherapeutic drugs have been obtained from retrospective studies. A randomized controlled trial would offer the strongest design to answer the remaining questions; however, long-term randomized studies are challenging with regards to logistics and financial feasibility, particularly in patients receiving chemotherapy.
While our study builds upon the current understanding of anthracycline- and TRA-induced cardiotoxicity by describing obesity as a possible protective factor, our study is limited as a single-center study, by the small sample size, and the infrequency of cardiotoxicity incidence. As only 16 out of 368 patients developed cardiotoxicity, it was a relatively rare outcome in our sample, which limited our statistical analysis. In the future, it will be critical to validate these findings with a larger, prospective study. Because of the small sample size, we were unable to include all hypothesized variables such as gender and race in the multivariate analysis due to their distribution. Therefore, the true effects of each of these predictors may not be fully identified due to limitations in our study’s statistical power to determine whether differences between groups truly exist. As a preliminary sample size analysis and power calculation were not performed, we cannot determine the true statistical power of the study. Additionally, as the information available was limited to the laboratory and imaging studies ordered by the treating medical team, our study may be skewed by reporting bias, as patients exhibiting overt symptoms of cardiotoxicity would be more likely to be evaluated with repeated serial testing or more specialized cardiac evaluation such as cardiac catheterization compared to asymptomatic patients. Finally, due to the design of the study, we were unable to ascertain the duration of observation of cardiac function and the percentage of patients for whom the observation period was completed.
Future efforts to further evaluate the association between obesity and cardiotoxicity should consider a case control study design or a hybrid design, as cardiotoxicity was a rare outcome and yielded insufficient statistical power in our cohort study design. Furthermore, although our study extended over a 5-year period of patient data, future studies should include expansion of study time periods to encompass a greater number of patients.
In conclusion, we report a study in which there was no association between obesity and the development of cardiotoxicity. Interestingly, based on the results, there is an indication that an increased BMI and/or being obese may be protective against cardiotoxicity, as increases in both BMI (kg/m2) and obesity had lower odds of cardiotoxicity. This finding is opposite of what was initially hypothesized. However, these findings were not statistically significant. Our recommendation is to regularly assess cardiac function in patients with anthracycline- or TRA-based chemotherapy and to carefully assess previous cardiac history and risk factors to stratify patient risk. Also, therapeutics aimed at preventing cardiac remodeling may be considered on a case-by-case basis. Future efforts to assess risk factors associated with anthracycline- and TRA-associated cardiotoxicity should be aimed at distinguishing the contribution of cardiovascular risk factors that may occur secondarily to obesity, including hypertension, from the independent impact of excess weight in overweight, obese, and severely obese patients.