Introduction
Amyloidosis is a rare and complex group of diseases characterized by the abnormal accumulation of protein deposits known as amyloids in various tissues and organs of the body. There are different types of amyloidosis, and the specific type is determined by the protein that forms the amyloid deposits [1]. ATTR cardiac amyloidosis can lead to heart failure, arrhythmias, and other heart-related outcomes. It is considered a rare disease, mainly affecting older adults, but the true prevalence of ATTR cardiac amyloidosis is hard to estimate, as it can vary significantly depending on various factors such as the specific type of ATTR amyloidosis, the region, and the population being studied [2–4]. Undoubtedly, cardiac amyloidosis is a serious disorder that requires specialized care. Timely diagnosis and appropriate management are essential to optimize outcomes and improve the patient’s prognosis. However, it is an underestimated health condition, whereas the exact paths and mechanisms that affect the cardiac system and risk of morbidity and mortality are not fully understood and appreciated.
Although diagnosis of cardiac amyloidosis typically involves a combination of tests, including echocardiography, cardiac magnetic resonance imaging (MRI), endomyocardial biopsy, as well as blood and urine tests [3], recently a simple ATTR-CM score has been introduced, the T-Amylo score, comprising six components, including age, sex, and echocardiographic measurements. The score has shown acceptable accuracy in specific heart failure populations (sensitivity 47%, specificity 94% and AUC 0.86, p < 0.001). However, the implementation of this score within the general population has never been examined before, especially in relation to morbidity and mortality due to cardiovascular disease (CVD). Employing the T-Amylo score within a general population context offers significant advantages in terms of early detection, public health research, personalized medicine, and health education, ultimately contributing to better health outcomes and disease management strategies. In the realm of personalized medicine, the T-Amylo score may offer tailored healthcare solutions. By incorporating individual risk factors and genetic information, healthcare providers can develop personalized prevention and treatment plans. This personalized approach enhances the effectiveness of medical interventions and improves patient adherence and satisfaction [4–8].
Thus, the aim of this study was to evaluate the associations between the T-Amylo score and a variety of anthropometric, biochemical, clinical, and echocardiographic indices related to CVD, as well as with CVD morbidity and mortality, in a general population, the Ikaria Island inhabitants.
Material and methods
Design
The Ikaria Study is an observational prospective, population-based study that was carried out on the island of Ikaria, northeast Aegean Sea, Greece, from June to October 2009. All residents of Ikaria aged 40 years old or older were asked to participate (population: 2,503; 2011 census).
Sampling procedure
The sampling was on a volunteer, convenience basis. Participants were interviewed by study’s trained personnel, i.e., cardiologists, general practitioners, nurses, and other healthcare professionals, who used a standard protocol and validated questionnaires. People living in institutions were excluded from the sampling.
Sample
Of the individuals asked to participate, 1,420 middle-aged and elderly inhabitants agreed to enroll in the study (participation rate 54%). The mean (standard deviation) age of the 678 male participants was 67 (14) years, and of the 742 females was 66 (14) years old. The studied sample was adequate to evaluate a two-sided hypothesis for odds ratios equal to 1.2, at a significance level of 0.05, achieving statistical power equal to 79.9% (NCSS and PASS software, Kaysville, Utah, 2004).
Measurements
Calculation of the T-Amylo score
The T-Amylo score, which was previously proposed and validated by Arana-Achaga et al., for the diagnosis of ATTR-CM, was calculated in this study for all participants [9]. In brief, Arana-Achaga et al. applied univariable logit models with a variety of clinical and biochemical parameters as covariates and the status of ATTR-CM as the outcome variable, using information retrieved from a multi-center study of heart failure patients. Continuous variables were dichotomized using clinically relevant thresholds, or by calculating the AUC and the relevant cut-off through ROC analysis. The regression coefficients of the final model became the weights of each parameter entered in the T-Amylo score. Each point was obtained considering the lowest beta coefficient; each weight was allocated based on the obtained coefficient rounded. Thus, a simplified score (i.e., T-Amylo score) was obtained assigning points as follows: 1 point for age ≥ 80 years, 2 points for IVSd thickness ≥ 16 mm, 2 points for low QRS interval voltage, 3 points for male gender, and 3 points for carpal tunnel syndrome.
The total score ranges from 0 to 11. In addition, a T-Amylo score cut-off point (i.e., score > 6) was used to classify patients as being at high risk for ATTR-CM (thereinafter high score), as proposed by Arana-Achaga et al. This cut-off was derived based on a sensitivity and specificity analysis [9].
Lifestyle characteristics
Current smokers were defined as those who smoked at least one cigarette per day during the past year; former smokers were defined as those who had stopped smoking for at least 1 year. The rest of the participants were defined as non-current smokers.
Physical activity was evaluated using the shortened version of the self-reported International Physical Activity Questionnaire (IPAQ) [10], which has been validated for the Greek population. Frequency (times per week), duration (minutes per time) and intensity of physical activity during sports, occupation and/or free-time activities were assessed. Participants who did not report any physical activities or reported a very low level (i.e., < 600 MET/min/week) were defined as physically inactive, while the rest were defined as at least minimally active.
Anthropometric measurements
Weight and height were measured following standard procedures and body mass index (BMI) was calculated in kg/m2. Obesity was defined as a BMI > 29.9 kg/m2. Waist circumference was measured at the midpoint between the bottom of the rib cage and the top of iliac crest from patients at minimal respiration. Body surface area (BSA), in m2, was calculated according to Mosteller equation (0.20247 × weight0.425 × height0.725) [11].
Biochemical measurements
For this work, several biochemical markers were retrieved from the bio-database of the Ikaria study and examined in relation to amyloid disease status [12–15]. In particular, levels of high-sensitivity C-reactive protein (hs-CRP) was assayed by particle-enhanced immune-nephelometry (N Latex, Date-Behring Marburg GmbH, Marburg, Germany). Interleukin-6 (IL-6) was measured with high-sensitivity enzyme-linked immunoassay (R & D Systems Europe Ltd, Abingdon, UK). Serum uric acid was determined using an enzymatic colorimetric test through the uricase-peroxidase method (UA plus, Roche Diagnostics, Manheim, Germany). Serum creatinine and urea were measured using a colorimetric method (BioAssay Systems, Hayward, CA, USA). Kidney function was evaluated by the creatinine clearance (CCr) rate, which is the volume of blood plasma that is cleared of creatinine per unit time. In particular, CCr was calculated using the Cockcroft-Gault (CG) formula [16]: CCr= [[(140 – age) × weight]/(72 × serum creatinine)] for males, while for females, the result of the above equation was multiplied by 0.85. Moreover, thyroid stimulating hormone (TSH) was also measured in mIU/ml to evaluate thyroid function.
Clinical measurements
Resting arterial blood pressure was measured three times in the right arm, at the end of the physical examination with subject in sitting position. Participants whose average blood pressure levels were greater than or equal to 140/90 mm Hg or were under anti-hypertensive medication were classified as having hypertension. Hypercholesterolemia was defined as total serum cholesterol levels higher than 200 mg/dl or the use of lipid lowering agents. Diabetes mellitus type 2 was determined by fasting plasma glucose tests and was analyzed in accordance with the American Diabetes Association diagnostic criteria (i.e., fasting blood glucose levels > 125 mg/dl or use of special medication, indicated the presence of diabetes). History of CVD (ICD-10 I25.0), i.e., acute myocardial infarction or angina, stroke (ICD-10 I63.9), heart failure (ICD-10 I50.9), or atrial fibrillation (ICD-10 I48.91), was evaluated by the cardiologists of the study, who also accessed participants’ medical files. Moreover, peripheral artery disease (ICD-10 I73.9), chronic obstructive pulmonary disease (ICD-10 J44.9), and thyroid disease (ICD-10 E07.9) were also evaluated through participants’ medical files.
Electrocardiographic measurements
Participants underwent a standard 12-lead ECG recording and a complete echocardiographic assessment; 195 subjects were excluded from the data analyses due to poor-quality ECG tracings (n = 88) or poor left ventricular M-mode echocardiographic tracings (n = 107). A resting 12-lead ECG was recorded during quiet respiration for each participant (duration 10 s) using SE-1010 PC ECG (EDAN instruments. Inc., Nanshan Shenzhen, China). Smart ECG Measurement and Interpretation Programs (SEMIP version 1.5), which is part of the EDAN SE series electrocardiograph and PC ECG system, was used for the automated measurement and interpretation of amplitudes and duration of ECG waves in each of the 12 leads. Adjustments of the automatically designated amplitudes and duration of ECG waves were performed by two blinded physicians of the study. From these measurements, seven ECG criteria were considered based on their general acceptance and recognized performance: five “pure voltage” criteria based on wave amplitude measurements, and two “time-voltage” criteria. The criteria were as follows: (1) Sokolow-Lyon voltage (sum of the amplitudes of S wave on V1 and R wave on V5 or V6 ≥ 3.5 mV) [15]; (2) sex-specific Cornell voltage (sum of the amplitudes of S wave on V3 and R wave on aVL > 2.0 mV in females and > 2.8 mV in men [16]; (3) Gubner-Ungerleider voltage (sum of the amplitudes of R wave on lead I and S wave on lead III ≥ 2.5 mV) [17]; (4) Lewis voltage (sum of the amplitudes of R wave on lead I and S wave on lead III, minus the amplitudes of S wave on lead I and R wave on lead III, ≥ 1.7 mV) [18]; (5) Framingham criterion (coexistence of a definite strain pattern and at least one of the following voltage criteria: sum of the amplitudes of the R wave on lead I and the S wave on lead III ≥ 2.5 mV, sum of the amplitudes of the S wave on lead V1 or V2 and the R wave on lead V5 or V6 ≥ 3.5 mV, the S wave on the right precordial lead ≥ 2.5 mV and the R wave on the left precordial lead ≥ 2.5 mV) [19]; (6) Sokolow-Lyon product (SV1 + RV5 or V6 × QRS duration ≥ 3000 mm.ms for females and ≥ 4000 mm.ms for males) [20]; and (7) Cornell product [(RaVL + SV3) + 8 mV for females] × QRS duration ≥ 2440 mm.ms) [20].
Cardiac ultrasonography
Standard transthoracic echocardiographic examination was carried out by the study’s physician in a dimly light room using a Vivid e cardiovascular ultrasound system (General Electric, Milwaukee, Wisconsin, USA) equipped with a 2.0 to 3.6 MHz (harmonics) phased-array transducer. The two-dimensional guided M-mode echocardiographic study of the left ventricle (LV) was performed at the parasternal long-axis view, and LV end-systolic and end-diastolic dimensions, as well as posterior wall and septal thicknesses, were measured as the mean from five consecutive cardiac cycles [21]. Reliability of the echocardiographic measurement of LV mass has been demonstrated in previous studies [22]. LV mass was calculated with the method of Devereux et al.: LV mass = 0.8 × 1.04 × [(LVID + VST + PWT)3 – LVID3] + 0.6, where LVID is left ventricular internal diameter, VST is ventricular septal thickness, and PWT is posterior wall thickness [23]. LV mass was indexed both for body surface area (BSA) and for height2.7. LVH was defined as LV mass indexed for BSA ≥ 125 g/m2 in males and ≥ 110 g/m2 in females [24] or LV mass indexed for height2.7 ≥ 49 g/m2.7 in males and ≥ 45 g/m2.7 in females [25].
Details about the assessment of biochemical, clinical and lifestyle characteristics of the Ikaria study’s participants have been extensively published elsewhere [12–14]. Further details about echocardiographic and ultrasonography methods applied in the Ikaria study participants may be found elsewhere [26].
Follow-up evaluation
Four years after first enrollment (in 2013), the study’s investigators performed a follow-up examination of the participants. Participants were appointed through telephone calls and then had face-to-face interviews with the study’s investigators.
The investigators performed a detailed evaluation regarding their:
vital status (death from any cause or due to CVD),
development of myocardial infarction, angina pectoris, other identified forms of ischemia (WHO-ICD coding 410-414.9, 427.2, 427.6-, heart failure of different types, and chronic arrhythmias – WHO-ICD coding 400.0-404.9, 427.0 -427.5, 427.9-), and
development of stroke (WHO-ICD coding 430-438).
All study participants underwent follow-up examination.
Statistical analysis
Continuous variables that followed a normal distribution are presented as mean (standard deviation (SD)), while T-Amylo score is presented as median (IQR) due to its skewed distribution. Categorical variables are presented as absolute and relative frequencies. The independent samples t-test and the non-parametric Mann-Whitney U-test were used for comparisons between means of normally or non-normally distributed continuous variables, respectively. Associations between categorical variables were tested by forming contingency tables and performing χ2 tests. Pearson or Spearman correlation coefficients were used, as appropriate, to test for correlations between continuous variables. Cox proportional hazards models were estimated to evaluate the association between T-Amylo score, all-cause mortality, and CVD-related outcomes, after various adjustments were made. Results are presented as hazard ratios (HR), along with their corresponding 95% confidence intervals. The log-rank test was used to compare survival curves during the follow-up. Furthermore, ROC analysis and the calculation of the area under the curve (AUC) were applied to evaluate the discriminative ability of the T-Amylo score in predicting combined CVD outcomes. To further evaluate the additive discrimination and reclassification ability of T-Amylo score for CVD outcomes in relation to its components, the net-reclassification improvement (NRI) and the integrated discrimination improvement (IDI) were also calculated (higher values suggest better performance of the predictive model). All reported p-values were based on two-sided hypotheses. All statistical calculations were performed using Stata statistical software (version 18.0; Texas, USA).
Results
Distribution of T-Amylo score
The median (IQR) T-Amylo score was 3.0 (3.0) and mean was 2.72 ±2.04. It was significantly higher in males as compared to females (median (IQR), 4.0. (3.0) vs. 2.0 (3.0), p < 0.001). When focused on participants aged 65 years or older, the mean T-Amylo score was 4.02 ±1.59 vs. 1.36 ±1.50 for the younger participants (p < 0.001). In addition, T-Amylo score was highly correlated with age, irrespective of gender of the participants (rho = 0.741, p < 0.001). The prevalence of participants with high risk (score > 6) in the entire sample was 9.6%; specifically, 120 (18.8%) males and 10 (1.4%) females had a T-Amylo score greater than 6 (p for gender differences < 0.001). For participants older than 65 years, this prevalence increased to 18.5% for both sexes. The actual prevalence of amyloidosis in the referent population was estimated to be 17% for males and 7% for females.
Univariate and multivariate associations between participants’ characteristics and high T-Amylo score
Tables I and II present participants’ characteristics ae according to the T-Amylo score levels. Participants with higher scores were older, more likely to be male, and had higher waist circumference and waist-to-hip ratio; however, differences in anthropometric indices became insignificant when age and gender were considered (p-values > 0.770). Moreover, participants with a high T-Amylo score were more likely to be ever smokers (p < 0.001), but this was explained (i.e., became insignificant, p = 0.554) when sex of the participants was considered, as males were 14 times more likely to be smokers compared to females (p < 0.001) (Table I).
Table I
Demographic and lifestyle characteristics of study participants, according to T-Amylo score of cardiac amyloidosis
Table II
Clinical, electrocardiographic, and echocardiographic characteristics of the study’s participants, according to T-Amylo score of cardiac amyloidosis
[i] Participants’ characteristics are presented as mean (standard deviation) and relative frequencies. P-values derived using independent samples t-test, or Mann-Whitney U-test (for non-normally distributed variables), or Pearson’s χ2 test. hs-CRP – high-sensitivity C-reactive protein, ECG-LVH – electrocardiography left ventricular hypertrophy, IL-6 – interleukin 6, LVMI – Left ventricular mass index, TSH – thyroid stimulating hormone, IVSd – interventricular septal end diastole, LVEDd – left ventricular end diastolic volume, PWT – posterior wall thickness.
Moreover, participants with a history of a CVD event were twice as likely to have a high T-Amylo score as compared to those without a history of CVD (95% CI: 1.23 to 3.32, p = 0.005), irrespective of age and gender. Higher prevalence of all CVD manifestations studied was observed among those with a high T-Amylo score as compared to those with a lower score. Similarly, participants with a high T-Amylo score were more likely to have a history of COPD but were less likely to have thyroid disease (Table II).
Regarding biochemical markers, participants with a high T-Amylo score had higher inflammation marker levels, as well as uric acid and creatinine levels, but they had a lower level of creatinine clearance (Table II). These associations of biochemical markers with high T-Amylo scores remained significant even after adjustments were made considering age, gender, smoking, and physical activity status, as well as BMI of the participants (p < 0.05).
All-cause and CVD-related mortality and morbidity during follow-up
During the follow-up, 53 deaths were observed, and thus, the mortality rate was 373 deaths per 10,000 inhabitants. Causes of death were myocardial infarction (21% of the cases), stroke (15% of the cases), cancer (21% of the cases), infection (10% of the cases), renal failure (4% of the cases), respiratory (3% of the cases), and the remaining 26% from other causes (e.g., accidents, etc.). Stratifying the analysis by T-Amylo score group, the distribution of cause of death was: in the low-score group, 18% myocardial infarction, 12% stroke, 20% cancer, 11% infection, 4% renal failure, 3% respiratory, and 25% from other causes; and in the high-score group, 22% myocardial infarction, 17% stroke, 21% cancer, 9% infection, 4% renal failure, 3% respiratory, and 27% from other causes. No significant differences were observed in the distribution of causes of death between T-Amylo score groups (all p > 0.20). Moreover, 134 participants (72 males, 22.6%, p for gender comparisons = 0.006) had a non-fatal CVD event (i.e., 45% acute myocardial infarction, 23% heart failure, 22% stroke, and 10% other manifestations of CVD) during the follow-up period (p for gender difference = 0.20). Stratifying the analysis by T-Amylo score group, the distribution of non-fatal CVD events was: in the low-score group, 44% myocardial infarction, 17% heart failure, 21% stroke, 18% other CVD; and in the high-score group, 47% myocardial infarction, 27% heart failure, 22% stroke, and 4% from other CVDs.
Assessment of T-Amylo score in relation to all-cause and CVD mortality and morbidity
The T-Amylo score was highly associated with all-cause mortality (HR = 1.59, 95% CI: 1.40 to 1.81), after adjusting for age, gender, physical activity and smoking status, BMI, history of previous CVD, as well as medical history of diabetes, hypertension, and hypercholesterolemia. This was evident in older participants (> 65 years), but not among younger participants (HR = 1.44, 95% CI: 1.22 to 1.70, and 1.16, 95% CI: 0.72 to 1.86, respectively).
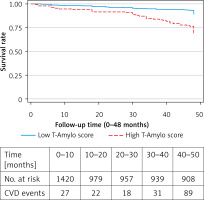
When focusing on combined CVD outcomes, participants with a high T-Amylo score had lower survival rates compared to those with a low score (p for log-rank test = 0.03) (Figure 1). In addition, T-Amylo score was positively associated with the risk of CVD events (fatal or non-fatal); in particular, a one-unit increase (1/11) in the score was associated with 32% higher risk for CVD (HR = 1.32, 95% CI: 1.11 to 1.56), after adjusting for age, gender, physical activity, smoking status, BMI, history of previous CVD, as well as medical history of diabetes, hypertension, and hypercholesterolemia. Then the analysis was repeated stratifying the sample into those < 65 and > 65 years old; it was observed that the T-Amylo score was not associated with younger age (HR = 1.21, 95% CI: 0.96 to 1.52), whereas it was highly associated with CVD events among older adults, as a one-unit increase was associated with 41% higher risk for CVD (HR = 1.41, 95% CI: 1.16 to 1.71), after the aforementioned adjustments were made.
Figure 1
Kaplan-Meier survival plot of T-Amylo score (high, ≥ 6/11 vs. low, < 6/11) for combined CVD events (fatal or non-fatal) during the 4-year follow-up (48 months) of n = 1,420 middle-aged and older participants of the Ikaria Study
Furthermore, to evaluate the classification ability of the T-Amylo score, ROC analysis was applied and revealed that the AUC was 0.70, and the accuracy of the score was 81.52%. The NRI and the IDI indices were 0.330 and 0.011 (p = 0.03), respectively, suggesting that the inclusion of T-Amylo score in the model that included the rest of parameters adds significantly to the correct classification of the model.
Significant interactions were observed between T-Amylo score, age, and sex of the participants (all p-values < 0.001). Thus, the analysis was stratified into participants below and above 65 years of age; it was observed that the AUC was 0.61, and the accuracy was 73.39%, for the younger participants, and the AUC was 0.83, and the accuracy was 92.25%, for the older participants. Moreover, we assessed the predictive value of the T-Amylo score stratifying by gender, and found that the AUC was 0.67 and the accuracy of the score was 77.78% in males; and the AUC was 0.77 and the accuracy of the score was 85.97% in females.
However, some of the parameters that are included in the T-Amylo score are well known to have an impact on CVD mortality and morbidity. Thus, we further explored the additive predictive value of the suggested score on CVD outcomes, as compared to its components. It was observed that compared to age, gender, IVSd thickness, QRS interval voltage, and carpal tunnel syndrome, the T-Amylo score improved the correct classification ability of the models by 4%, 7%, 8%, 5%, and 5%, respectively (IDI, all p-values < 0.001).
Discussion
The aim of this study was to evaluate the associations between a cardiac amyloidosis score, the T-Amylo score, with CVD morbidity and mortality and its related predictors, in a population known for longevity. Transthyretin amyloid cardiomyopathy is often found in patients with heart failure with preserved ejection fraction (HFpEF). Previous studies have demonstrated the clinical value of the T-Amylo score for ATTR-CM in selected populations, i.e., patients with diagnosed heart failure [27]. The data analysis of approximately 1,400 apparently healthy, middle-aged individuals from the general population revealed that the ATTR-CM related score, i.e., the T-Amylo score, seemed to act as a good discriminator in predicting all-cause mortality and CVD events. The predictive value of the score was independent of the presence of obesity, smoking status, history of diabetes mellitus, arterial hypertension, chronic obstructive pulmonary disease, and thyroid disease, but it was moderated by older age and female sex of the participants. Moreover, the T-Amylo score improved the correct classification ability of the models that included solely its components, i.e., age, gender, IVSd thickness, QRS interval voltage, and carpal tunnel syndrome, by 4% to 8%; suggesting that a considerable number of CVD patients could have been identified by using this score.
In line with the findings of a previous study which applied the T-Amylo score in HFpEF patients [9], in this study, individuals with a low T-Amylo score (i.e., score < 6), compared to those with high score, were older, more likely to be male, and had a lower body mass index, left ventricular ejection fraction, and estimated glomerular filtration rate. In addition, in other relevant studies in patients with clinically diagnosed HFpEF that have also incorporated ATTR-CM scores, such as the Mayo ATTR-CM score, subjects with a high score had lower prevalence of hypertension, but a higher prevalence of atrial fibrillation and coronary artery disease. Additionally, it was reported that the ATTR-CM scores were associated with increased risks of CVD outcomes, as well as all-cause mortality, and hospitalization due to heart failure [27–30]. In this study, we expanded the previous findings to the general population by showing that the T-Amylo score for ATTR-CM is significantly associated with all-cause and CVD mortality and morbidity. However, it should be noted that these associations were more pronounced among older adults than in younger ones.
However, the question arises why the score is not as effective in individuals younger than 65. It may be that the factors weighted heavily in the score are less prevalent or significant in younger individuals. Moreover, age, septal thickness, and gender, all components of the T-Amylo score, are significant predictors in older populations but may not capture the nuanced risk factors relevant to younger individuals. Additionally, many subjects with increased septal thickness might have hypertension, a condition that is more common and impactful in older adults.
As patients with ATTR-CM experience a severe, progressive disease, a greater understanding of the presentation and progression of ATTR-CM can guide physicians in earlier diagnosis and treatment of patients with ATTR-CM. Even beyond screening for ATTR-CM, higher values of ATTR score are significantly correlated with the existence of CVD, chronic obstructive pulmonary disease, thyroid disease, lower creatinine clearance, higher IL6 levels, male sex, and lower body weight. This was also evident in the TOPCAT study’s sub-analyses, where compared with patients with a low ATTR-CM score, those with a high ATTR-CM score were, older, more likely to be male, and had lower body mass index, blood pressure, LVEF, and estimated glomerular filtration rate. In addition, patients with a low ATTR-CM score had lower prevalence of hypertension, but a higher prevalence of atrial fibrillation and coronary artery disease [27].
Furthermore, higher scores were associated with the presence of LVH, while, independently of this relationship, higher ATTR-CM scores were associated with higher risk of adverse clinical outcomes. This is in line with the findings of this study and illustrates the difficulty to detect ATTR-CM in individuals with many comorbidities; in whom even symptoms may be undetected. Thus, simplified scores have significant prognostic value, as they are significantly associated with cardiovascular events even when applied in a general population without a known heart failure diagnosis.
The T-Amylo score has been designed and tested in selected populations and not in a general population, as in the present study; although this might be a limitation, this is also a novelty of this study. The diagnosis of amyloidosis requires a multidisciplinary approach, combining clinical assessment, laboratory tests, imaging studies, and tissue biopsies. By implementing the T-Amylo score in the general population, early detection and intervention efforts are enhanced, as laboratory testing, imaging, and genetic studies can be difficult to implement in daily clinical practice in identifying cardiac ATTR amyloidosis. Recognizing individuals at an elevated risk for amyloid-related conditions allows for timely preventive measures, potentially slowing disease progression or even preventing onset of the disease. In addition, the T-Amylo score provides a standardized, objective measure that can be broadly applied, making it a valuable tool for public health initiatives. Its use in population-wide screening programs can facilitate large-scale epidemiological studies, yielding insights into the prevalence and distribution of amyloid-related diseases. The general population’s use of the T-Amylo score also supports personalized medicine approaches. By integrating individual risk profiles into healthcare planning, clinicians can tailor recommendations for lifestyle modifications, monitoring, and treatment plans, thereby optimizing patient outcomes. Moreover, the widespread application of the T-Amylo score can contribute to raising public awareness about amyloid-related diseases. As individuals become more informed about their health risks, they may be more proactive in seeking medical advice and adopting healthier behaviors.
The sampling procedure may introduce potential selection bias and healthy volunteer effect, as people living in institutions were excluded from the Ikaria study, and the general participation rate was 54%. For evaluating all-cause and CVD mortality and morbidity in a general population, the sample of 1420 subjects is not adequate to be considered as representative of the general population, also taking into account that the follow-up was only of 4 years duration. The M-mode was used for the LV measurements according to the former ESC guidelines (2005); however, the current guidelines recommend 2D measurements for LV dimensions. In the clinical diagnosis of ATTR-CM, several echocardiographic indices are also used, e.g. longitudinal left ventricular strain, and other multiparametric scores that incorporate more advanced echocardiographic features, to increase the diagnostic accuracy, which were not available in this study. Thus, to avoid confusion, and due to the lack of relevant validation of the T-Amylo score in the general population, no diagnosis of ATTR-CM is presented here.
In conclusion, as the general population is getting older, and the predisposing factors for CVD in older individuals are not well understood and appreciated, the application of markers for the prognosis of CVD morbidity and mortality through uncovering other predisposing health states is of major importance. In this study, the prognostic value of an ATTR-CM score, the T-Amylo score, was evaluated and found promising for prognosis of CVDs in the general population.