Introduction
Prostate cancer (PCa) ranks second among the most prevalent cancers in men and is the second leading cause of male cancer-related mortality. It has been projected that in 2040, there will be 2.9 million new cases of PCa globally [1, 2]. The likelihood of a man being diagnosed with PCa at some point in his lifetime is notably high, reaching 11.2% [3]. While established risk factors for PCa include age, race, family history, and smoking, there is currently a lack of identified modifiable risk factors [3]. Presently, approximately 90% of patients exhibit localized tumor progression at the time of PCa diagnosis, rendering surgical treatment inappropriate. Therefore, early detection and effective intervention of PCa are crucial in enhancing patient prognosis [4–6].
Inflammation is widely acknowledged as a crucial factor in the aging process and age-related diseases, including heart disease, diabetes, and Alzheimer’s disease, with tumor development being particularly affected. This association has led to the recognition of a concept termed “inflamm-aging”, which describes the occurrence of chronic, low-grade inflammation that progressively increases with age. Inflamm-aging is attributed to the persistent exposure to antigens and stressors, which, over time, establish a continuous presence of inflammatory stimuli that contributes to the susceptibility to age-related diseases and disabilities. Many epidemiological studies investigating inflammation rely on a single biomarker, typically C-reactive protein (CRP), interleukin-6 (IL-6), or tumor necrosis factor-α (TNF-α), to quantify the inflammatory state. In PCa, inflammation plays a crucial role in the disease’s pathophysiology, influencing its onset and prognosis.
Previous research has indicated a link between PCa risk and inflammatory cytokines such as MCP-1 and IL-4. However, due to the intricate nature of the inflammatory system and its involvement in various feedback mechanisms, relying solely on a single inflammatory marker may explain the inconsistent findings reported in the literature. Moreover, whether inflammatory cytokines act as initiating factors or are downstream effects of poor functional outcomes in PCa remains a topic of debate and requires further investigation.
Mendelian randomization (MR) analysis employs single nucleotide polymorphisms (SNPs) as genetic instrumental variables (IVs) to proxy for exposure factors [7, 8]. By comparison to observational studies, MR analysis mitigates the effects of reverse causation and confounding factors on the findings, thereby yielding more robust evidence for causal inference [9].
In order to evaluate the potential correlation between circulating inflammatory cytokines and PCa, and to determine the direction of this correlation, we conducted a bidirectional two-sample MR analysis. Initially, we obtained validated genetic IV for 91 inflammatory cytokines from genome-wide association study (GWAS) data, and proceeded to analyze their association with PCa. Additionally, we explored the causal relationship by reversing the exposure and outcome variables. This study contributes to the existing evidence supporting the targeting of specific inflammatory cytokines for the purpose of PCa prevention.
Material and methods
We employed bidirectional two-sample MR to evaluate the causal relationship between inflammatory cytokines and PCa. All data utilized in this study were obtained from publicly available GWAS summary statistics, thereby obviating the need for additional ethical approval or informed consent. We conducted a thorough search for GWAS summary statistics and extracted primary SNPs that were linked to inflammatory cytokines or PCa as genetic IV. The GWAS summary statistics for PCa comprised of 9,132 cases and 173,493 controls, and could be accessed from the MRC IEU GWAS Database (https://gwas.mrcieu.ac.uk/datasets/ieu-b-4809/). The GWAS summary statistics for cytokines were sourced from the GWAS Catalog platform, available at GWAS Catalog (https://www.ebi.ac.uk/gwas/publications/37563310). Ethical approval was obtained from the FinnGen steering committee for all selected GWASs in the FinnGen Consortium, and individuals provided written informed consent.
Selection of IV for inflammatory cytokines
The IVs utilized for inflammatory cytokines were obtained from a recent meta-analysis GWAS, which enrolled 14,824 individuals of European ancestry from 11 cohorts. These participants were subjected to measurement of 91 inflammatory cytokines using the Olink Target Inflammation panel. To identify SNPs associated with the levels of these inflammatory cytokines in circulation, a genome-wide significance level (p < 5 × 10–8) was employed. Subsequently, the summary data of the SNPs were extracted, and linkage disequilibrium (LD) SNPs were pruned by applying an r2 threshold of < 0.1. The SNP with the lowest p-value was selected as an independent instrument. Herein, we identified 91 SNPs that exhibited significant associations with inflammatory cytokines (p-value < 5 × 10–8, LD r2 < 0.01, clump = 1000 kb). To evaluate the efficacy of the remaining SNPs, the F-statistic was computed for each SNP (F = beta2/se2), and an overall F-statistic was determined as well. The overall F-statistic was calculated as 169.6, surpassing the typical threshold of 10, thereby indicating a robust ability of genetic variants to predict inflammatory cytokine levels.
PCa GWAS summary data
We employed the PCa GWAS summary data obtained from the MRC Integrative Epidemiology Unit (MRC-IEU) Consortium (ID: ieu-b-4809). This dataset consisted of 182,625 individuals of European ancestry, encompassing 9,132 cases and 173,493 controls with PCa. A comprehensive collection of 9,851,867 SNPs was included in this investigation. The summary statistics can be readily accessed and downloaded from the consortium’s website. It is important to underscore that all these data are de-identified, publicly available, and can be utilized without any constraints.
Statistical analysis
This study used inverse variance weighting (IVW) as the primary methodology for MR analysis. To evaluate the heterogeneity among SNPs [10], the Cochran Q-test was employed. Additional analyses were conducted using the weighted median and MR-Egger methods. The MR-Egger intercept test was used to assess horizontal pleiotropy, while MR-PRESSO was used to detect outlier SNPs [11, 12]. These identified SNPs were subsequently excluded from the MR analysis to ensure the reliability and consistency of the results. A leave-one-out analysis was conducted to evaluate the robustness of the findings. Furthermore, supplementary sensitivity analyses were performed using the weighted mode and simple mode analyses [10]. In cases where the IVW analysis yielded statistically significant results (p < 0.05) and no evidence of horizontal pleiotropy or heterogeneity was found, the findings could be considered positive, even if the other methods did not yield significant results, as long as the direction of the β-values remained consistent. When horizontal pleiotropy was present without heterogeneity, the MR-Egger method was used [13]. In situations where heterogeneity was observed without the presence of pleiotropy, either the weighted median method or the random effects IVW method was utilized. The analysis was performed using R 4.2.2 software and the R packages “TwosampleMR” and “MR-PRESSO” [10, 13].
Results
Causal association of inflammatory cytokines with PCa
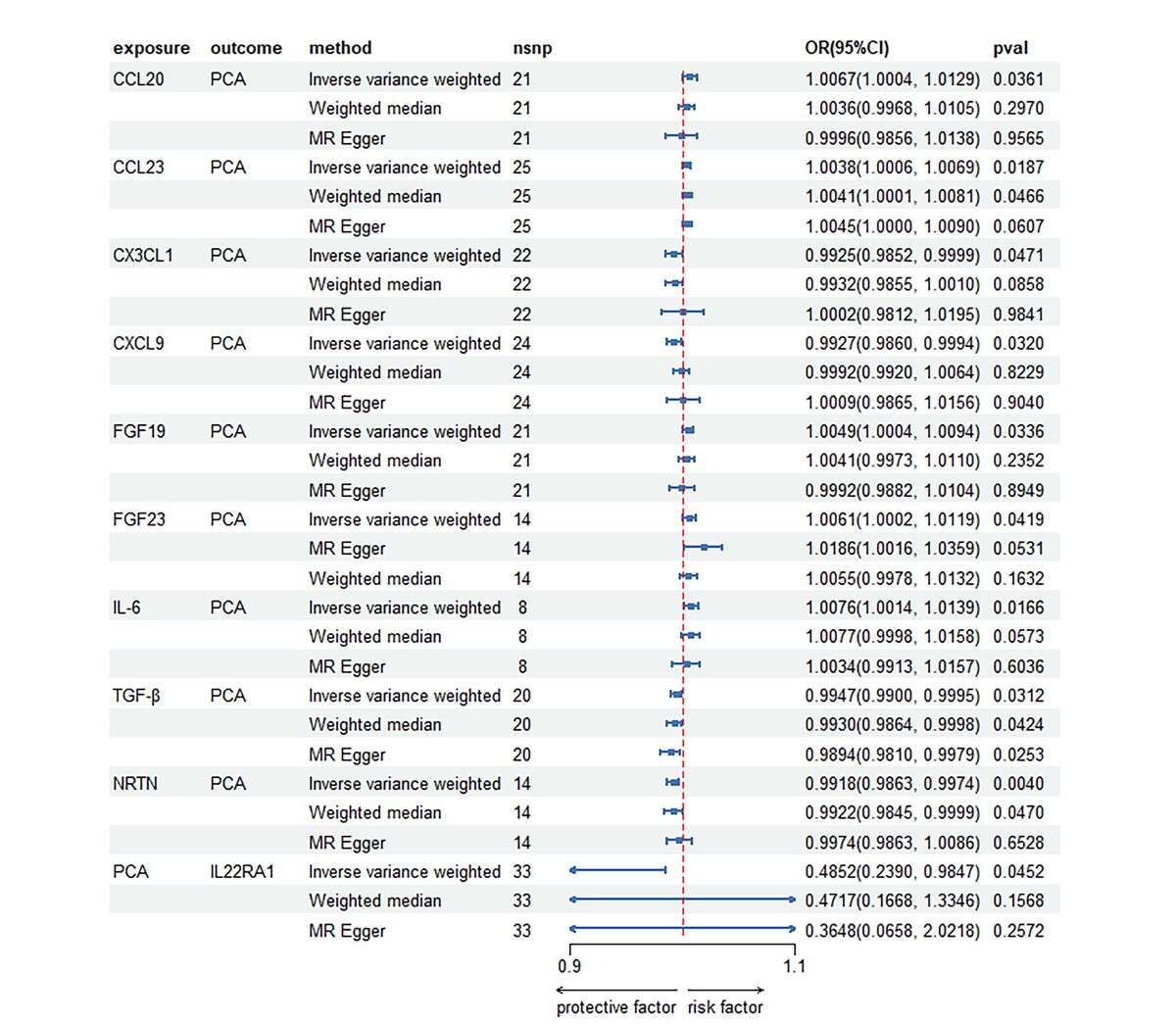
After performing a series of quality control procedures, a total of 33 SNPs were identified as being associated with PCa, while 169 SNPs were found to be associated with inflammatory cytokines (Table I, Supplementary Tables SI, SII). Regarding the inflammatory cytokines, the number of SNPs per cytokine protein varied from 8 to 33. All included SNPs had F values greater than 10. Following the Bonferroni correction, the analysis using the IVW method revealed evidence supporting an association between 9 inflammatory cytokines and PCa (Supplementary Table SIII). Of the known inflammatory cytokines, 7 showed a positive correlation with PCa, whereas 2 exhibited a negative correlation. Among the positively correlated inflammatory factors were levels of CCL20, CCL23, FGF19, FGF23, and IL-6. The strongest positive association effect was observed for IL-6 (OR [95% CI]: 1.0076 [1.0014, 1.0139]), followed by CCL-20 (OR [95% CI]: 1.0067 [1.0004, 1.0129]) and FGF23 (OR [95% CI]: 1.0061 [1.0002, 1.0119]).
Table I
Causal associations between significant cytokine traits and prostate cancer estimated by different two-sample MR methods
Several inflammatory factors showed a negative correlation with PCa, including CX3CL1 (fractalkine levels), CXCL9 (C-X-C motif chemokine 9 levels), TGF-β (latency-associated peptide transforming growth factor beta 1 levels), and NRTN (neurturin levels). Among these factors, NTRN had the strongest negative association effect, with an odds ratio (OR) of 0.9918 and a 95% confidence interval (CI) of 0.9863 to 0.9974 (Table I; Figures 1, 2).
Using PCa as an exposure to test bidirectionality of associations
After conducting multiple test corrections, we consistently found evidence from the IVW method indicating a negative causal effect of PCa on IL22RA1 (interleukin-22 receptor subunit alpha-1 levels) with an OR (95% CI) of 0.4852 (0.2390, 0.9847). However, the weighted median method yielded an OR (95% CI) of 0.4717 (0.1668, 1.3346), while the MR Egger method indicated an OR (95% CI) of 0.3648 (0.0658, 2.0218). The p-values for both these methods were greater than 0.05, suggesting that the supplementary methods were unable to confirm the causal effect. These results are summarized in Table I, Supplementary Table SIV, Figures 1, and 2.
Sensitivity analysis
Sensitivity analyses were conducted to assess the robustness of the above results. Cochran’s Q test of the IVW method and MR Egger method was used. The results showed that only CCL20 (MR Egger Q p-value: 0.0342, IVW Q p-value: 0.0289), CX3CL1 (MR Egger Q p-value: 0.0013, IVW Q p-value: 0.0012), and CXCL9 (MR Egger Q p-value: 0.0008, IVW Q p-value: 0.0004) exhibited heterogeneity of IV variables (p < 0.05) and were significantly associated with PCA. The other inflammatory cytokines did not show significant heterogeneity. In terms of reverse MR, no heterogeneity of PCA affecting inflammatory cytokines was observed. Moreover, the MR-Egger regression intercept analysis revealed no apparent horizontal pleiotropy in the bidirectional MR analysis. The direction of effect in all three methods was consistent with the IVW method. The radial MR results of IVW indicated that the corrected results were consistent with the uncorrected ones. Furthermore, the results remained almost identical to the overall MR effect value after excluding each SNP one by one through leave-one-out analysis, indicating strong robustness of the results (Table I; Supplementary Tables SV; SVI; Figure 3).
Discussion
This study utilized a two-sample bidirectional MR analysis approach to examine the causal association between inflammatory cytokines in the bloodstream and PCa. In the initial phase, the study evaluated the causal association between 91 circulating inflammatory cytokines, encompassing chemokines, interleukins, and growth factors, and the occurrence of PCa. The findings revealed a positive correlation between genetically predicted elevated levels of CCL20, CCL23, FGF19, FGF23, and IL-6, and PCa outcomes. Furthermore, considering PCa as an exposure variable, it appeared to elicit a decrease in IL22RA1 levels via pathogenic mechanisms.
CCL20 and CCL23 are chemokines that play a role in promoting the directed migration of leukocytes, T cells, eosinophils, and basophils to sites of injury or inflammation [14]. These chemokines act as pro-inflammatory molecules in diseases. Inflammatory response is a critical pathological process in PCa, and the systemic inflammatory response mediated by CCL20 and CCL23 exacerbates the delay in the resolution of inflammation and worsens the infiltration and progression of PCa tissues [15]. Observational studies have also provided evidence of the association between CCL20, CCL23, and the risk and functional prognosis of PCa. A prospective study has indicated that higher circulating levels of CCL20 and CCL23 can predict the risk of PCa. Therefore, CCL20 and CCL23 are influential factors in the risk of PCa and may also contribute to the post-PCa inflammatory pathological changes [16].
The FGF signaling pathway has been demonstrated to play a crucial role in the progression of PCa [17]. FGF19, a member of the FGF subfamily, has been shown to circulate in the serum and exert its effects in an endocrine manner. A recent study investigated the impact of FGF19 on the proliferation and epithelial-mesenchymal transition (EMT) of PC3 cells, shedding light on the association between the presence of FGF19-positive cells in the tissues of patients with PCa. This research provides evidence that FGF19 may contribute to the development of PCa by promoting cell proliferation and EMT in PCa.
FGF23 is an endocrine FGF, primarily expressed by osteocytes. It plays a crucial role in maintaining phosphate homeostasis by engaging in feedback loops with the kidneys and vitamin D [18, 19]. A study demonstrated that FGF23 functions as an autocrine growth factor in all cell types. It was observed that supplementation with exogenous FGF23 increases cell proliferation, invasion capabilities, and anchorage-independent growth in vitro. Conversely, knockdown of FGF23 in PCa cell lines reduces these phenotypes. Furthermore, suppressing FGF23 expression also results in a decrease in tumor growth in vivo [20]. These findings suggest that FGF23 may function as an autocrine, paracrine, and/or endocrine growth factor in PCa, thereby contributing to disease progression [20].
Another study confirmed the role of IL-6 in the pathogenesis of PCa. Through an evaluation of the expression of IL-6 superfamily members, related cytokines, and the potential involvement of IL-6 in regulating PCa growth [21, 22], the study demonstrated that IL-6 appears to experience a functional shift from acting as a paracrine growth inhibitor to functioning as an autocrine growth stimulant during the progression of prostate cancer towards a hormone-resistant phenotype [23].
Our findings from reverse MR analysis indicate that PCa may be associated with a decrease in the inflammatory level of IL-22RA1. Interleukin-22 (IL-22) is a member of the IL-10 cytokine family and is produced by T cells and innate lymphoid cells. The IL-22 signaling pathway plays a crucial role in coordinating mucosal immune defense and tissue regeneration. Its pleiotropic actions include pro-survival signals, cell migration, developmental anomalies, and angiogenesis [24]. These functions contribute to the prevention of initial tumor formation. The involvement of the IL-22/IL-22RA1 axis in cancer is intricate. Evidence suggests that IL-22 expression and signaling are dysregulated in patients with various common cancers such as intestinal, skin, lung, and liver cancer. Although IL-22 displays limited tissue specificity, its receptor IL-22R1 is exclusively expressed on epithelial and tissue cells. Nonetheless, it is an attractive therapeutic target on immune cells due to its potential to achieve anti-tumor immunity with reduced side effects [24]. Our study further supports this notion by demonstrating that PCa occurrence is associated with a decrease in the inflammatory level of IL-22RA1, thereby highlighting it as a promising therapeutic target.
Our study has several limitations. Firstly, all the GWAS summary statistics used in our study were derived from participants of European ancestry, which may limit the generalizability of our findings to other ethnicities. Secondly, our study included 91 cytokines, while several other important inflammatory biomarkers were not included due to the lack of IVs available for analysis. Additionally, the results for various cytokines such as IFN-γ, IL-5, and IL-13 were based on a single genetic variant, which might lead to lower precision. Thirdly, due to the assumptions of aggregate data level MR analysis in our study, we were unable to investigate the non-linear effects of cytokines and growth factors on PCa. Fourthly, the genetic estimates of cytokines were derived from GWAS adjusted for body mass index (BMI). Although the associations remained consistent when using unweighted allele scores, which minimizes bias in the genetic association estimates of exposures, we cannot exclude the possibility that adjustment for BMI may have led to collider bias during instrument selection. Obesity has been identified as a significant comorbidity in prostate cancer. Studies have shown that obesity is associated with high-grade and aggressive prostate cancer, as well as increased mortality [25]. This relationship can be partly attributed to low-grade inflammation, where cytokines and chemokines play a crucial role. Elevated levels of inflammatory markers in obese individuals may contribute to the progression and severity of prostate cancer. Fifthly, the associations found in MR analysis are based on genetic SNPs, representing the cumulative effect of exposure over an individual’s lifetime. It is possible that exposure to cytokines only affects PCa during specific time windows. Lastly, due to the unavailability of information on disease aggressiveness or molecular subtypes, we focused solely on the association between cytokines and the overall risk of PCa. Nevertheless, our study provides new insights into the relationship between inflammatory biomarkers and PCa, enhancing our understanding of its etiology [10, 12].
In summary, this study utilizing MR analysis has several notable advantages. First, it minimizes confounding factors and reverse causation, providing robust causal inference about the relationship between inflammatory cytokines and prostate cancer. Second, the use of large-scale GWAS data enhances the statistical power and reliability of our findings. Third, by identifying specific inflammatory cytokines such as CCL20, CCL23, FGF19, FGF23, and IL-6 as being associated with an elevated risk of PCa, our study offers supportive evidence of a causal relationship, indicating that these cytokines could serve as potential targets for therapeutic interventions in PCa. Additionally, the study suggests that IL22RA1 might have the potential to serve as a biomarker for monitoring therapeutic effects in advanced stages of PCa. These findings not only provide new insights into the etiology of prostate cancer but also open up new avenues for preventive and clinical strategies. This two-sample MR study examines the genetic correlation and bidirectional relationship between inflammatory activity and PCa using large-scale GWAS data. The findings reveal that genetically predicted elevated levels of CCL20, CCL23, FGF19, FGF23, and IL-6 are positively associated with PCa outcomes, whereas elevated levels of CX3CL1, CXCL9, TGF-β, and NRTN are negatively associated with PCa outcomes. Additionally, the inverse MR results suggest that the presence of PCa may lead to a decrease in inflammatory levels of IL-22RA1. This finding implies that PCa expression could serve as a potential indicator for identifying patients who are likely to respond to immunotherapy, further offering potential targets for novel immunotherapeutic approaches. Overall, this study provides novel insights into PCa biomarkers and pathways, with important implications for PCa prevention and clinical intervention.
Our findings have several potential clinical implications. By identifying specific inflammatory cytokines such as CCL20, CCL23, FGF19, FGF23, and IL-6 as associated with an elevated risk of prostate cancer, our study suggests that these cytokines could serve as biomarkers for early detection and risk stratification in clinical settings. Furthermore, targeting these cytokines through therapeutic interventions could provide novel approaches for preventing and treating prostate cancer. The observed decrease in IL22RA1 levels associated with prostate cancer suggests that IL22RA1 might be a valuable biomarker for monitoring therapeutic effects, particularly in advanced stages of the disease. Overall, our research highlights the importance of understanding the inflammatory pathways involved in prostate cancer, paving the way for improved clinical outcomes through targeted interventions.
In conclusion, this study suggests a positive correlation between elevated levels of CCL20, CCL23, FGF19, FGF23, and IL-6 and the risk of prostate cancer. Additionally, prostate cancer appears to causally reduce IL22RA1 levels. These findings provide new insights into potential biomarkers and therapeutic targets for prostate cancer prevention and treatment. Our research highlights the importance of targeting specific inflammatory cytokines and understanding their bidirectional relationships with prostate cancer to develop effective clinical interventions.