Introduction
Acute ischemic stroke is the most common type of stroke, accounting for 80% of all strokes [1], with high rates of disability, mortality and recurrence [2]. Non-valvular atrial fibrillation, a serious risk to human health, increases the risk of stroke by 5 times [3]. Vitamin K antagonist (warfarin), the mainstay of anticoagulant therapy in patients with atrial fibrillation, has been shown to reduce the risk of stroke by 65% [4]. However, warfarin is not convenient for use in clinical practice due to a series of defects such as a narrow therapeutic window, large individual differentiation, frequent monitoring, and the influence of diet [5]. With the emergence of non-vitamin K oral anticoagulants that avoid many shortcomings of vitamin K antagonists, prevention of ischemic stroke has entered an exciting era. Apixaban, a novel anticoagulant known as a factor Xa inhibitor, has been shown in many studies to be more effective than warfarin in preventing stroke and reducing the risk of bleeding [6]. However, a 2014 meta-analysis [7] by Ruff et al. showed that the non-vitamin K oral anticoagulants increased gastrointestinal bleeding by 25% compared to warfarin. This result may be due to high heterogeneity of the included studies, which were caused by unclear dosage, medication methods and follow-up time. In the 2017 network meta-analysis [8] of López-López et al., compared to warfarin and other non-vitamin anticoagulants (such as dabigatran, edoxaban, and rivaroxaban), apixaban was ranked not only as the most effective intervention among the evaluation results, but also as the safest. Therefore, the purpose of this meta-analysis is to further confirm the efficacy and safety of apixaban, to provide an evidence-based reference for clinical diagnosis and treatment.
Material and methods
Search strategy
PubMed, Embase, Cochrane Library, Web of Science, Clinical Trials, CNKI, CBM, and Wanfang databases were searched by computer, and the retrieval time was from establishment to May 31 2021. The following search terms were used: (apixaban OR Eliquis OR BMS 562247 OR BMS562247 OR BMS-562247) AND (Warfarin OR 4-Hydroxy-3-(3-oxo-1-phenylbutyl)-2H-1-benzopyran-2-one OR Apo-Warfarin OR Aldocumar OR Gen-Warfarin OR Warfant) AND (Ischemic Stroke OR Ischemic Strokes OR Stroke, Ischemic OR Ischaemic Stroke OR Ischaemic Stroke). At the same time, we reviewed the references in the articles we retrieved for more reports to avoid missing them in the search [9–12].
Inclusion criteria
(1) Populations: all patients with non-valve atrial fibrillation; (2) Interventions: the use of apixaban in the trial group; (3) Control: the use of warfarin in the control group; (4) Outcome indicators: effectiveness indicators including the incidence of ischemic stroke, any thrombosis events, and safety indicators including major bleeding, cerebral hemorrhage, and gastrointestinal bleeding; (5) Study designs: randomized controlled trial or well-designed cohort; (6) Follow-up for at least 3 months interviews.
Exclusion criteria
(1) Summaries, letters, comments, case reports and editorials of meetings, (2) studies with incomplete data and inaccessible data, (3) studies published repeatedly, (4) animal studies.
Data extraction
Two reviewers independently screened the literature according to the inclusion and exclusion criteria, downloaded the full text of literature that met the criteria and read them carefully, screened them again and cross-checked them. In case of disagreement, the literature was resolved through discussion or adjudicated with the assistance of a third party. After that, data were extracted independently according to the predesigned data extraction table (Table I). The extracted information was as follows: first author, publication year, data source country, sample size, patients’ baseline, specific interventions, HAS-BLED score, CHADS2 score, CHADS2-VASc score, follow-up time, outcome indicators, and dose.
Table I
Major characteristics of studies included in this meta-analysis
Quality assessment
The risk of bias in randomized controlled trials was assessed using the modified Jadad scale [13], which includes a total score of 7 points, with 1–3 being low quality and 4–7 being high quality. The Newcastle-Ottawa Scale [14] (NOS) was used to assess the risk of bias in the included cohort studies [9–12, 15–21], as detailed in Table II. A score of 7–9 points represents high quality and a lower score represents low quality research. Thus, of the 10 cohort studies, 7 were considered to be of high methodological quality (low risk of bias), while the other 3 were considered to be of low methodological quality (high risk of bias), with scores below 7.
Table II
Risk of bias assessment of the included studies according to the modified Newcastle-Ottawa Scale (NOS)
Statistical analysis
The RevMan 5.3 statistical software provided by the Cochrane Collaboration was used for statistical analysis. Mean difference (MD) was used as the statistic of effect analysis. Odds ratio (OR) was used as the statistic of effect analysis for categorical variables, and 95% confidence intervals (CI) was used for interval estimation. If there was no statistical heterogeneity among results (p > 0.10, I2 ≤ 50%), a fixed effects model was used for meta-analysis. If not, a random effects model was used for meta-analysis. If the heterogeneity of the analysis results remained high or the sources of heterogeneity could not be identified, a descriptive sensitivity analysis was conducted to identify the source of heterogeneity by removing individual studies one by one. Forest plots were used to determine whether the difference between apixaban and warfarin in the treatment of ischemic stroke was statistically significant. Funnel plots were used to assess publication bias of included studies [9–12, 15–21].
Results
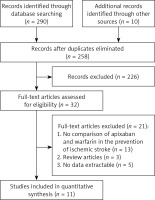
Included literature
After an initial review, 300 publications were obtained, and 32 publications remained after excluding duplicates and irrelevant literature. After exclusion of non-conforming literature, 11 studies were retained, including 6 retrospective cohort studies, 4 observational studies, and 1 randomized controlled trial. In total the literature concerned 240,652 patients, including 86,595 in the apixaban group and 154,057 in the warfarin group. The literature screening process and results are shown in Figure 1. The basic characteristics of the included literature are shown in Table I.
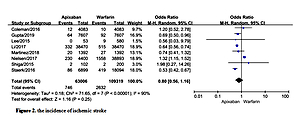
Incidence of ischemic stroke
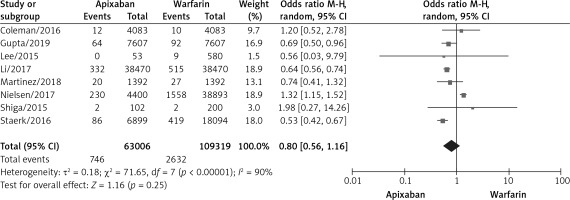
A total of 8 studies documented ischemic stroke [9–12, 16, 17, 20, 21] with statistical heterogeneity among the studies (p < 0.00001, I2 = 90%). A random effects model was used for the meta-analysis, as shown in Figure 2. The results of the meta-analysis showed that the incidence of ischemic stroke was not statistically significantly different between the two groups (OR = 0.80, 95%CI (0.56, 1.16), p = 0.25).
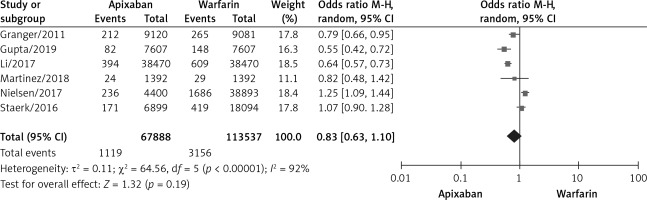
Incidence of any thromboembolic event
A total of 6 studies documented thromboembolic events [10–12, 15, 17, 21] with statistical heterogeneity among studies (p < 0.00001, I2 = 92%). A random effects model was used for the meta-analysis, as shown in Figure 3. The results of the meta-analysis showed no statistically significant differences between the apixaban and warfarin groups (OR = 0.83, 95% CI (0.63, 1.10), p = 0.19).
Incidence of major bleeding
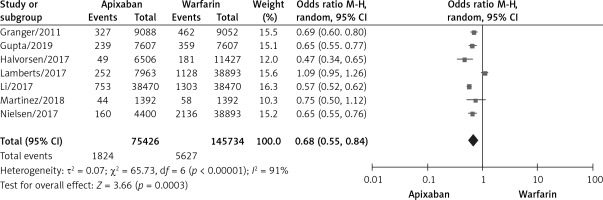
A total of 7 studies documented major bleeding [10–12, 15, 17–19], with statistical heterogeneity among studies (p < 0.00001, I2 = 91%). A random effects model was used for the meta-analysis, as shown in Figure 4. The results of the meta-analysis showed that apixaban significantly reduced the incidence of massive bleeding compared with warfarin, and the difference was statistically significant (OR = 0.68, 95% CI (0.55, 0.84), p = 0.0003).
Incidence of intracranial hemorrhage
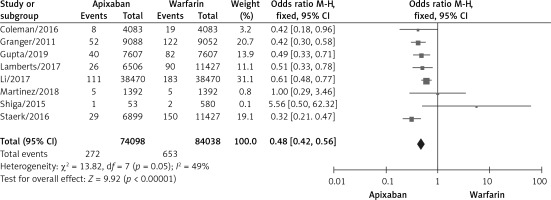
A total of 8 studies documented intracranial hemorrhage [10, 11, 15–17, 19–21], with no significant statistical heterogeneity among the studies (p < 0.00001, I2 = 92%). A fixed effects model was used for the meta-analysis, as shown in Figure 5. The results of the meta-analysis showed that apixaban significantly reduced the incidence of intracranial hemorrhage compared with warfarin, and the difference was statistically significant (OR = 0.48, 95% CI (0.42, 0.56), p < 0.00001).
Incidence of gastrointestinal hemorrhage
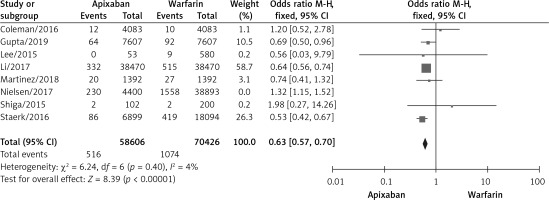
A total of 6 studies documented gastrointestinal bleeding [10, 11, 15, 17, 18, 20], and no statistically significant heterogeneity was found among the studies (p = 0.11, I2 = 44%). A fixed effects model was used for the meta-analysis, as shown in Figure 6. The results of the meta-analysis showed that compared with warfarin, apixaban significantly reduced the incidence of gastrointestinal bleeding, with statistical significance (OR = 0.66, 95% CI (0.60, 0.72), p < 0.00001).
Sensitivity analysis
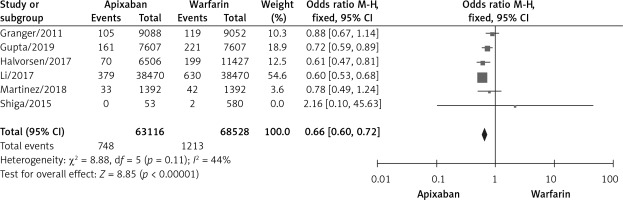
The meta-analysis suggested a high degree of heterogeneity in the incidence of ischemic stroke, any thromboembolic events and major bleeding, so we performed sensitivity analysis (Figure 7). Among the indicators of hemorrhage incidence, there was no significant difference in the results after removing the literature on a case-by-case basis. In a sensitivity analysis of the incidence of ischemic stroke and the incidence of any thromboembolic events, Nielsen’s study was found to be a source of heterogeneity. After excluding Nielsen’s study, the results of our meta-analysis showed that apixaban significantly reduced the incidence of ischemic stroke [9–11, 16, 17, 20, 21] (OR = 0.63, 95% CI (0.57, 0.70), p < 0.00001) and the incidence of any thromboembolic events [10, 11, 15, 17, 21] (OR = 0.75, 95% CI (0.59, 0.96), p = 0.02).
Publication bias
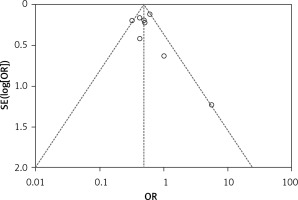
An inverted funnel plot was drawn with the incidence of intracranial hemorrhage as an indicator. Overall, there was no obvious asymmetry in the funnel plots of the 8 included studies. Although there were some differences in sample size between the two groups of studies, most of the included studies had large sample sizes, so the results of our meta-analysis were relatively reliable, as shown in Figure 8.
Discussion
Although there have been some meta-analyses comparing the efficacy of apixaban and warfarin at home and abroad, these studies included fewer than 10 papers and had large heterogeneity. For example, in Pan et al.’s meta-analysis [22] including four trials, apixaban was more effective in reducing intracranial hemorrhage (HR = 0.47; 95% CI: 0.24–0.92, p = 0.0001 for heterogeneity, I2 = 86%). In Proietti et al.’s meta-analysis [23] including nine trials, apixaban was more effective in reducing any thromboembolic event (OR = 0.77; 95% CI: 0.64–0.93, p = 0.02 for heterogeneity, I2 = 62%). However, our meta-analysis screened all trials comparing the efficacy and safety of apixaban and warfarin over 10 years, included 11 studies and has high quality scores of the included literature, which indicates sufficient credibility.
In our analysis, we found high heterogeneity in the incidence of ischemic stroke. Then we conducted a related sensitivity analysis, suggesting a significant decrease in heterogeneity after excluding Nielsen’s article [12] (p = 0.40, I2 = 4%), and the remaining 7 studies [9–11, 16, 17, 20, 21] after excluding Nielsen’s study were analyzed according to a fixed effects model, showing that apixaban significantly reduced the incidence of stroke compared to warfarin (OR = 0.63, 95% CI (0.57, 0.70), p < 0.00001). However, the sample size of Nielsen’s research was large and Nielsen’s study was rated as high quality by NOS. One reason for the high heterogeneity in the incidence of ischemic stroke in our meta-analysis may be that the patients in Nielsen’s research were treated with a low dosage (2.5 mg twice a day) of apixaban, whereas most studies were based on higher dosages or the dosage was based on a comprehensive evaluation by medical personnel (such as based on patients’ age, infirmity, fragility of blood vessels, and comorbidity, etc.).
While in the ARISTOTLE trial [15], apixaban significantly reduced the risk of stroke or systemic embolism, major bleeding, intracranial hemorrhage, and all-cause mortality compared to warfarin, and was also better tolerated, our meta-analysis showed no significant difference in real-life stroke and thrombotic events compared with warfarin, but apixaban was safer than warfarin, and in particular was associated with a lower incidence of intracranial bleeding, the most disastrous complication of anticoagulants. The cause might be that the ARISTOTLE trial was a randomized controlled trial (RCT), and our meta-analysis included 10 cohort studies. Therefore, more RCT studies are needed.
It is well known that long-term warfarin anticoagulation has become the gold standard for treatment to prevent thrombotic complications for patients using left ventricular assist devices. However, Whitehouse et al. [24] compared adverse events of apixaban and warfarin in patients receiving the HeartMate 3 LVAD and found no significant difference in safety, so apixaban is a possible alternative and may be better in compliance, and no additional testing is required. In the future, more and more patients will use apixaban instead of warfarin.
In our research we made great efforts to obtain relatively objective results. First, this meta-analysis is the most comprehensive collection of reports in this field and represents the most complete analysis of apixaban in the prevention of ischemic stroke. Second, two experienced researchers independently evaluated and extracted all data from the study, including reducing potential publication bias. The results showed that compared with warfarin, apixaban significantly reduced the risk of massive bleeding, intracranial bleeding and gastrointestinal bleeding, which can be widely used in clinical practice.
Although the present study has achieved positive results, there may be deficiencies that should not be ignored, as follows: (1) The period ranged from 3 months to several years, so long-term efficacy and safety could not be evaluated. (2) Only Chinese or English studies were included, which may entail some publication bias. (3) A subgroup analysis based on apixaban dosage was not conducted in our current analysis. In future analyses, with more and more trials, it will be important to evaluate the efficacy and safety of apixaban according to dosages (for example, apixaban 2.5 mg BID or apixaban 5 mg BID versus warfarin, respectively). (4) There is no specific antidote or reversal agent for apixaban. In case of overdose, oral administration of activated charcoal is recommended to absorb drugs from the gastrointestinal tract. If uncontrolled bleeding occurs, prothrombin complex concentrate, activator VII, or fresh frozen plasma (factor Xa inhibitor) may be helpful. It is hoped that effective reversal strategies for various non-vitamin K oral anticoagulants will be developed in the future. (5) The pharmacological action of apixaban is dose-dependent and the anticoagulant effect is affected by the level of drug exposure [25]. For female patients, patients with low body weight (< 50 kg), elderly patients (> 65 years old) and patients with liver and kidney damage, the plasma exposure of apixaban will be increased, along with an increased risk of bleeding. There is no evidence in clinical practice on how to adjust the dosage of apixaban. However, we should provide personalized treatment according to different patients, taking their disease development into comprehensive consideration. It is hoped that relevant studies will be better conducted in the future. (6) Because apixaban is a novel anticoagulant, the cost of treatment is higher and more difficult for low-income families, which prevents its widespread use to some extent.
In conclusion, compared with warfarin, apixaban showed no statistically significant difference in efficacy but a significant difference in safety, significantly reducing the risk of major bleeding, intracranial bleeding, and gastrointestinal bleeding. Apixaban has clinical advantages in the prevention of ischemic stroke, and is worthy of widespread clinical promotion.