Introduction
Eosinophil cationic protein (ECP), also known as ribonuclease3 (RNase3), is a basic protein located in the eosinophil primary matrix [1]. ECP is encoded by the RNase3 gene, released during eosinophilic degranulation, and related to asthma and airway inflammation [2]. ECP is largely responsible for damaging the bronchial mucosa, where eosinophilic infiltration occurs during asthmatic inflammation process [3]. During the progression of the inflammatory process, ECP released locally in the inflamed mucosa is permeated to the circulation and has a systemic impact [4].
ECP is a potent cytotoxic protein that inhibits cell viability and induces cellular apoptosis [5]. However, there have been inconsistent reports regarding the cytotoxicity caused by ECP. In one study, ECP increased tumour necrosis factor-α (TNF-α) production and triggered apoptosis via caspase activation [6], whereas in another study, lymphoma cell line treated with ECP revealed a characteristic feature of necrotic cell death [7].
APO-1 (CD95), designated as Fas molecule, is a cell surface death receptor expressed on many cells including eosinophils, which can lead to apoptosis induction when triggered by agonistic antibodies or their ligands [8]. A soluble form of APO-1 is produced via proteolytic cleavage of membrane-bound receptors or alternative splicing [9]. TNF-related apoptosis-inducing factor (TRAIL) is a death ligand, a member of the TNF/nerve growth factor superfamily. TRAIL can induce apoptosis in a broad range of human cancer cell lines. TRAIL selectively kills cancer cells without causing any harm to normal cells and is thereby a prospective candidate for cancer therapeutic strategies [10].
The role of ECP as a biomarker of airway inflammation and as a causal factor in allergic respiratory diseases has been extensively studied. However, few studies have closely examined the relationship between ECP, APO-1, and TRAIL in allergic diseases. In the present study, our hypothesis is that apoptotic activity is more closely associated with the severity of systemic inflammation than serum ECP and immunoglobulin E (IgE) levels in patients with allergic diseases. To test the hypothesis of this study, serum soluble apoptotic markers in allergic diseases and their relationships with ECP, allergen-specific IgE (sIgE), total IgE (tIgE), and inflammatory parameters were investigated.
This study also examined which of these variables more critically contributes to apoptotic activity, particularly focused on sensitisation to food and inhalant allergens.
Material and methods
Study population
A total of 125 patients were evaluated, who were diagnosed with allergic diseases between 2017 and 2019 at Inha University Hospital. Their age ranged from 21 to 65 years (mean age: 43.5 years), with 67 patients of the male sex (53.6%). Age- and sex-matched healthy individuals (n = 30), who had no evidence of allergic diseases and atopic sensitisation, were enrolled as the control group. Several variables including ECP, APO-1, TRAIL, tIgE, sIgE, and high-sensitivity C-reactive protein (hsCRP) levels were measured. Body mass index (BMI) and systolic blood pressure were measured because these variables can be potential sources of bias in assessing inflammation level. The patients had the following diseases: acute bronchial asthma (n = 61), food allergy (n = 29), allergic rhinitis (n = 23), atopic dermatitis (n = 7), allergic conjunctivitis (n = 3), and anaphylaxis (n = 2). The study population with confirmed allergy was recruited based on the following criteria: a clinical history of allergic diseases, positive sIgE test results (inhalant and/or food allergens determined according to the ImmunoCAP test), and elevated serum tIgE level. The exclusion criteria were as follows: subjects with medication history including corticosteroids or histamine antagonists, acute infection with fever (> 37.5°C), chronic illness, and recent operation. The study protocol was approved by the institutional review board, and written informed consent was obtained from all participants. This study was conducted in accordance with the guidelines of the Helsinki Declaration.
Measurement of ECP, sIgE, and tIgE
Blood samples were collected from patients during their first visit to the hospital, immediately centrifuged, and stored at –70°C until analysis. All specimens were obtained before treatment. The serum ECP level was measured by performing a chemiluminescent immunometric assay using an Immulite 2000 analyser (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). A medical decision level for serum ECP results was defined as 19 µg/l based on the manufacturer’s instruction. Serum concentration of sIgE was tested using a fluoroenzyme immunoassay (ImmunoCAP 100, Phadia AB, Uppsala, Sweden) for identifying two of the most common inhalant allergens (Dermatophagoides pteronyssinus and Dermatophagoides farinae) and two major food allergens (egg white and cow’s milk). An sIgE level of ≥ 0.35 kU/l (≥ class 1) was considered to be indicative of allergic sensitisation [11]. The sIgE score was defined as the sum of the class (0 to 6), which was assigned to each patient according to the degree of allergic sensitisation. Serum tIgE concentrations were analysed via an immunoradiometric assay (Coat-A-Count Total IgE IRMA, Siemens Healthcare Diagnostics, Tarrytown, NY, USA).
APO-1, TRAIL, and inflammatory parameters
APO-1 and TRAIL concentrations were measured via enzyme immunoassays performed using human APO-1/FasBMS245 kits (Bender MedSystems, Vienna, Austria) and Quantikine human TRAIL/TNFSF10 kits (R&D Systems, Minneapolis, MN, USA), respectively. Serum hsCRP level was analysed by performing a particle-enhanced immunonephelometry assay using a chemical analyser (Hitachi 7600; Hitachi, Tokyo, Japan). An elevated hsCRP level was defined at > 0.5 mg/dl, which was based on the cut-off value of the 95% confidence interval for hsCRP levels in healthy individuals. Blood eosinophil counts and absolute neutrophil counts were estimated using an automated analyser (ADVIA 120; Siemens, Forchheim, Germany).
Patient categorisation
Patients were categorised into two groups based on ECP and sIgE: patients with a positive test result for ECP (> 19 µg/l; n = 104) and those with a negative test result for ECP (≤ 19 µg/l; n = 21), as well as patients with inhalant allergy (n = 96) and those with food allergy (n = 29). Patients who tested positive for ECP were further stratified into two groups according to hsCRP concentration: patients with an elevated hsCRP level (> 0.5 mg/dl; n = 80) and those without an elevated hsCRP level (≤ 0.5 mg/dl; n = 24).
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation, and non-normally distributed data were presented as median (interquartile range). The normality of data distribution was tested using the Shapiro-Wilk test. Categorical variables were expressed as frequencies and percentages. Continuous variables with normal distribution were analysed using Student’s t-test. Non-normally distributed data were analysed using the Mann-Whitney U-test. Categorical variables were analysed using the χ2 test. A multivariate regression analysis for elucidating the relationship between eosinophil activation and apoptosis was conducted after adjusting for potential confounders, such as age, sex, BMI, heart rate, and systolic blood pressure. Data were analysed using SPSS software (IBM SPSS Statistics for Windows, version 19.0. Armonk, NY, USA). A p-value of < 0.05 was considered to be statistically significant.
Results
Clinical and laboratory characteristics of subjects
ECP, tIgE, and hsCRP levels were significantly higher in patients with allergic diseases than in the control subjects. Serum APO-1 and TRAIL concentrations in patients with allergic diseases were 420.8 pg/ml and 85.7 pg/ml, respectively, which were significantly higher than those in healthy individuals (217.3 pg/ml and 42.9 pg/ml, respectively, p < 0.001). Of the 125 patients, 104 (83.2%) had an elevated ECP level and 91 (72.8%) had an elevated hsCRP concentration (Table I).
Table I
Clinical and laboratory characteristics of the study population
Clinical presentation of patients with allergic diseases
Of the 125 patients with allergic diseases, 96 (76.8%) had inhalant allergy and 29 (23.2%) had food allergy. Among the total patients, 67 (53.6%) had a history of allergic attack, including shortness of breath, chest tightness, swelling of the airway, wheezing, or a sudden decrease in blood pressure. In the patients with allergic diseases, median duration of allergic diseases was 1.2 years, and the frequency of allergic attack was 2.3 times per year (Table II).
Table II
Clinical presentation of patients with allergic diseases
Apoptotic activity in allergic diseases
Serum tIgE and hsCRP levels were significantly higher in patients who tested positive for ECP than in those who tested negative for ECP. However, there were no significant differences in serum APO-1 and TRAIL concentrations between the two groups. The sIgE levels for inhalant allergens were significantly higher in patients who tested positive for ECP than in those who tested negative for ECP. However, sIgE levels for food allergens did not differ between the groups. Of the 104 patients who tested positive for ECP, 80 (76.9%) had an elevated hsCRP level, which was a significantly higher proportion than the 52.3% (11/21) of patients who tested negative for ECP (p = 0.021) (Table III).
Table III
Apoptotic markers and inflammatory parameters in relation to ECP levels in patients with allergic diseases
Apoptosis in patients with systemic inflammation
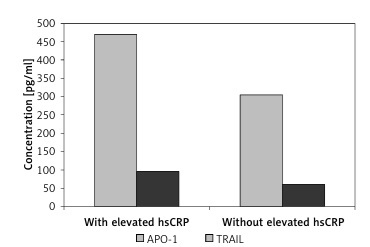
APO-1 and TRAIL concentrations in relation to serum hsCRP level in patients who tested positive for ECP are shown in Figure 1. Patients with an elevated hsCRP level exhibited significantly higher APO-1 and TRAIL concentrations than those without an elevated hsCRP level (469.1 pg/ml and 95.3 pg/ml vs. 305.4 pg/ml and 61.2 pg/ml, respectively, p < 0.001) (Figure 1).
Figure 1
APO-1 and TRAIL concentrations in patients who tested positive for ECP (n = 104) in relation to hsCRP levels. APO-1 and TRAIL concentrations are significantly higher in subjects with elevated hsCRP levels (n = 80) than in subjects without elevated hsCRP levels (n = 24) (469.1 pg/ml and 95.3 pg/ml vs. 305.4 pg/ml and 61.2 pg/ml, respectively, p < 0.001)
APO-1 and TRAIL in relation to the type of allergy
Patients with inhalant allergy exhibited significantly higher levels of ECP, APO-1, and TRAIL than those with food allergy (54.7 µg/l vs. 32.8 µg/l, p < 0.001; 453.9 pg/ml vs. 312.1 pg/ml, p = 0.016; 91.2 pg/ml vs. 64.5 pg/ml, p = 0.023). Serum hsCRP concentrations were two times higher in patients with inhalant allergy than those in patients with food allergy (0.83 mg/dl vs. 0.41 mg/dl, p < 0.001) (Table IV).
Table IV
APO-1, TRAIL, and inflammatory parameters in relation to the type of allergy
Effect of ECP and hsCRP on apoptotic activity
The effect of ECP and hsCRP on apoptotic markers was evaluated. After excluding the patients with elevated ECP levels from the study subjects, APO-1 and TRAIL levels still remained higher in the patients than in healthy individuals (358.4 pg/ml and 69.5 pg/ml vs. 217.3 pg/ml and 42.9 pg/ml, respectively, p < 0.05). However, after excluding the patients with elevated hsCRP levels from the study subjects, no significant difference was observed in APO-1 and TRAIL levels between the two groups (Table V).
Table V
Serum concentrations of APO-1 and TRAIL after excluding patients with elevated ECP and hsCRP levels
Relationship between apoptotic markers, ECP, hsCRP, and types of allergens
After adjusting for potential confounders, serum APO-1 and TRAIL levels were positively associated with ECP (r = 0.32 and r = 0.29, respectively, p < 0.001) and hsCRP (r = 0.34 and r = 0.31, respectively, p < 0.001). Serum ECP levels were positively correlated with hsCRP levels, tIgE, and sIgE scores (r = 0.32, r = 0.37 and r = 0.26, respectively, p < 0.001 for all). There was no significant association between APO-1 and tIgE or sIgE scores, nor between TRAIL and tIgE or sIgE scores (p > 0.05 for all). Inhalant allergens were significantly associated with ECP and hsCRP levels (r = 0.24 and r = 0.23, respectively, p < 0.001); however, food allergens had no significant association with the corresponding parameters (Table VI).
Table VI
Regression analysis for elucidation of the relationship between soluble apoptotic markers and ECP, hsCRP, and types of allergens in patients with allergic diseases
Discussion
In the present study, the relationships between apoptotic markers, ECP, sIgE, and hsCRP were assessed in patients with allergic diseases. APO-1 and TRAIL levels were significantly higher in patients with allergic diseases than in healthy subjects, demonstrating a positive relationship with ECP concentration. The results of this study revealed that apoptosis is enhanced in allergic diseases; however, apoptotic activity is elevated only when augmented ECP production is accompanied by systemic inflammation.
ECP and tIgE are reportedly increased and closely associated with each other in allergic diseases. However, conflicting results have been reported for ECP and tIgE [12]. Several studies demonstrated that there were no significant associations between ECP and serum tIgE levels in children with asthma or urticaria [13, 14]. Additionally, serum ECP concentrations were unexpectedly low in asthmatic patients [15]. Contrastingly, in another study, tIgE was significantly related to ECP in patients with allergic rhinitis [16]. In our study, ECP levels were positively correlated with tIgE and sIgE scores. These inconsistencies may reflect the differences in both the immune response of patients and eosinophil activation in various pathologic conditions between the present and previous studies.
Bronchial asthma is characterised by chronic airway inflammation caused by immune cells. A study revealed that hsCRP level was significantly elevated in patients with asthma attacks and was related to the state of asthma exacerbation [17]. Sileem et al. [18] demonstrated that serum concentrations of hsCRP and tIgE significantly increased in asthmatic patients compared with those in healthy individuals. In our study, 72.8% of allergic patients showed an increase in hsCRP concentration. These results implied that a considerable number of allergic patients develop systemic inflammation. These findings may be attributable to an enhanced production of a potent cytotoxic ECP, which is released into systemic circulation by activated eosinophils.
Apoptosis is categorised into caspase-dependent and caspase-independent types [19, 20]. Caspase-dependent apoptosis is classified into three pathways: mitochondria-, endoplasmic reticulum-associated, and death receptor-initiated pathways [21]. Several studies have reported that ECP induces apoptosis via mitochondria-independent pathway [22] and causes cytotoxicity via caspase-like activity [23, 24]. However, the exact mechanism of ECP-induced apoptosis remains unclear. A recent study demonstrated that recombinant ECP mainly induces the necrosis of bronchial epithelial cells [25].
In this study, serum soluble apoptotic markers were evaluated in relation to serum ECP levels. There were no significant differences in APO-1 and TRAIL levels between the groups of patients with positive and negative tests for ECP. However, among the patients who tested positive for ECP, patients with elevated hsCRP levels exhibited significantly higher APO-1 and TRAIL concentrations than those without elevated hsCRP levels. Moreover, APO-1 and TRAIL were more closely associated with hsCRP than tIgE and sIgE scores. These results suggest that apoptosis is more strongly induced by concomitant systemic inflammation than by atopic sensitisation or by a mere elevation in ECP level. These findings are in accordance with the results of previous studies, which demonstrated that the level of nucleosomes, a soluble parameter for apoptosis, is significantly elevated during systemic inflammation [26, 27].
Most of the allergic sensitisation initially occurs against food allergens, and the sensitisation is gradually directed against inhalant allergens [28]. Prolonged exposure to food allergens can be a major risk factor for the development of allergic airway diseases [29]. APO-1 and TRAIL levels have not yet been investigated in association with specific sensitisation to food and inhalant allergens. The current study tested the difference in apoptotic activity according to the types of allergens. APO-1 and TRAIL levels were significantly higher in patients who tested positive for inhalant allergens than in those who tested positive for food allergens. A possible explanation for these findings is that inhalant allergens are more prone to induce systemic inflammation than ingested allergens. In fact, in the current study, serum hsCRP concentrations were two times higher in patients with inhalant allergy than in those with food allergy. Furthermore, inhalant allergens were positively associated with ECP and hsCRP levels; however, food allergens had no significant association with the corresponding parameters. These results suggest that inhalant allergens play a more important role in systemic inflammation and apoptotic regulation than food allergens.
This study examined which parameter between augmented ECP production and systemic inflammation more critically contributes to apoptotic markers in allergic diseases. When patients with elevated ECP levels were excluded from the study subjects, serum APO-1 and TRAIL concentrations were found to still be higher than the serum APO-1 and TRAIL concentrations in the control subjects. However, when patients with elevated hsCRP levels were excluded from the subjects, their serum APO-1 and TRAIL concentrations decreased to levels similar to those in the control subjects. After excluding the patients with high hsCRP levels from the study population, elevated APO-1 and TRAIL concentrations were restored to levels similar to those in healthy individuals, suggesting that concurrent systemic inflammation strongly contributes to apoptotic activity in allergic diseases.
This study has several limitations. Serum ECP levels in serial samples were not measured for assessment of the changes in ECP in association with disease progression. The sIgE levels were assessed for only four specific allergens. Non-normally distributed variables may influence multivariate regression analysis, although the analysis was conducted after adjusting for potential confounders. The time frame for collecting blood samples may differ among the subject populations, particularly in regard to the exact time of an ongoing allergic attack, although blood specimens were obtained from patients during their first visit to the hospital due to severe allergic reactions. Despite these limitations, this study has considerable significance. To our knowledge, the present study shows, for the first time, the association of ECP and APO-1 and TRAIL levels, particularly in relation to food and inhalant allergens. However, further validation is warranted through larger randomized prospective studies.
In conclusion, this study demonstrates that APO-1 and TRAIL are closely linked to hsCRP and ECP but not to tIgE levels and sIgE scores. Serum APO-1 and TRAIL levels were significantly higher in allergic patients with elevated hsCRP levels than in those without elevated hsCRP levels. These results suggest that enhanced apoptotic activity has crucial implication with concomitant systemic inflammation than with the intensity of atopic sensitisation, especially in patients with inhalant allergy.