Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ANDROLOGY / CLINICAL RESEARCH
Genetic causal associations between serum metabolites and infertility: a Mendelian randomization study
1
Department of Urology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, China
2
Department of Obstetrics and Gynecology, Jingjiang People’s Hospital Affiliated to Yangzhou University, Taizhou, China
3
Department of Obstetrics and Gynecology, The First Affiliated Hospital of Soochow University, Suzhou, China
These authors had equal contribution to this work
Submission date: 2024-10-28
Final revision date: 2025-03-24
Acceptance date: 2025-04-22
Online publication date: 2025-06-08
Corresponding author
Tian Tao
Department of Obstetrics and Gynecology The First Affiliated Hospital of Soochow University Suzhou, China
Department of Obstetrics and Gynecology The First Affiliated Hospital of Soochow University Suzhou, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
This study aimed to elucidate the causal relationships between serum metabolites and infertility in both men and women, and to identify key metabolic biomarkers.
Material and methods:
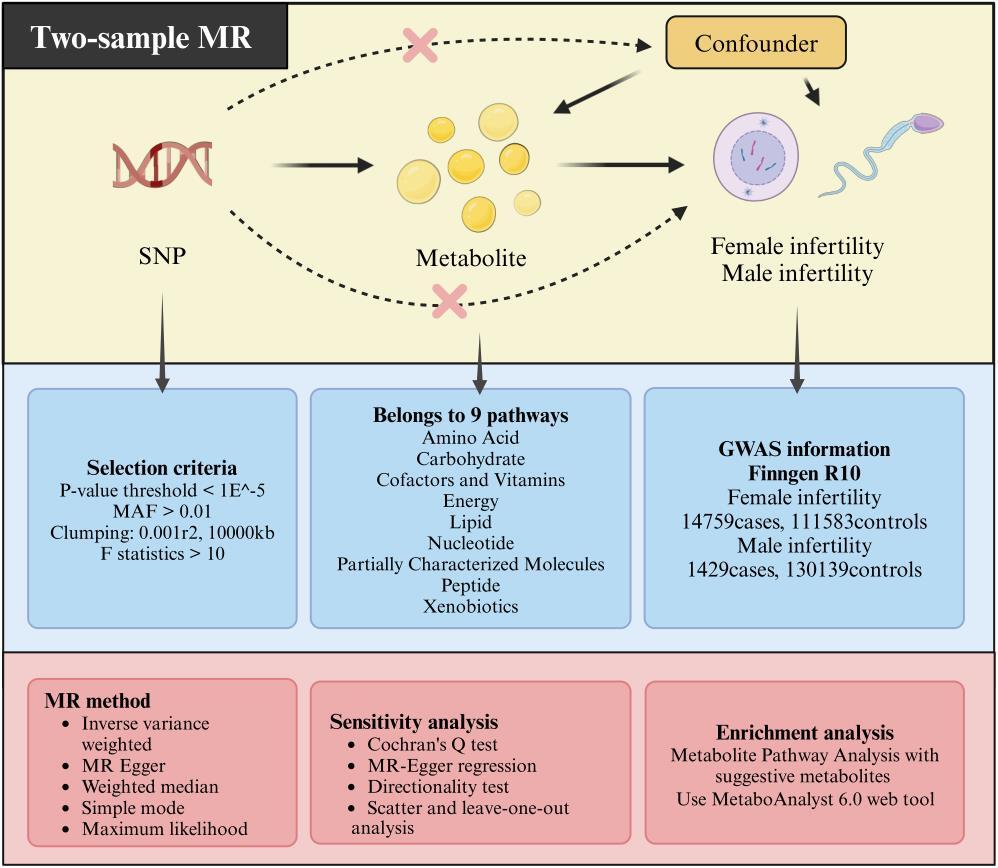
This study employed a two-sample Mendelian randomization design, with circulating plasma metabolite genome-wide association study data as an exposure factor and FinnGen Consortium R10 genome-wide association study data for infertility in men and women as an outcome. The causal relation between plasma metabolites and infertility in men and women was assessed using five methods: inverse variance weighted, Egger regression, weighted median, maximum likelihood estimation, and simple mode.
Results:
This analysis identified 17 and 10 metabolites positively and negatively associated with infertility in women, respectively. Similarly, 22 and 30 metabolites were positively and negatively associated with infertility in men, respectively. Galactonate and glycerate levels were identified as risk factors for infertility in both men and women. In addition, sphingomyelin exerts protective effects against infertility in both men and women. Metabolic pathway analysis revealed enrichment of critical metabolic pathways related to infertility.
Conclusions:
This study identified several circulating metabolic biomarkers associated with infertility. These biomarkers can be used for the screening and prevention of infertility. In addition, they could be employed as candidate molecules for future mechanistic exploration and drug-targeting studies.
This study aimed to elucidate the causal relationships between serum metabolites and infertility in both men and women, and to identify key metabolic biomarkers.
Material and methods:
This study employed a two-sample Mendelian randomization design, with circulating plasma metabolite genome-wide association study data as an exposure factor and FinnGen Consortium R10 genome-wide association study data for infertility in men and women as an outcome. The causal relation between plasma metabolites and infertility in men and women was assessed using five methods: inverse variance weighted, Egger regression, weighted median, maximum likelihood estimation, and simple mode.
Results:
This analysis identified 17 and 10 metabolites positively and negatively associated with infertility in women, respectively. Similarly, 22 and 30 metabolites were positively and negatively associated with infertility in men, respectively. Galactonate and glycerate levels were identified as risk factors for infertility in both men and women. In addition, sphingomyelin exerts protective effects against infertility in both men and women. Metabolic pathway analysis revealed enrichment of critical metabolic pathways related to infertility.
Conclusions:
This study identified several circulating metabolic biomarkers associated with infertility. These biomarkers can be used for the screening and prevention of infertility. In addition, they could be employed as candidate molecules for future mechanistic exploration and drug-targeting studies.
REFERENCES (30)
1.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem 2018; 62: 2-10.
2.
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Med 2012; 9: e1001356.
3.
Service CA, Puri D, Al Azzawi S, Hsieh TC, Patel DP. The impact of obesity and metabolic health on male fertility: a systematic review. Fertil Steril 2023; 120: 1098-111.
4.
Dai M, Hong L, Yin T, Liu S. Disturbed follicular microenvironment in polycystic ovary syndrome: relationship to oocyte quality and infertility. Endocrinology 2024; 165: bqae023.
5.
Kovac JR, Pastuszak AW, Lamb DJ. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril 2013; 99: 998-1007.
6.
Zhang A, Sun H, Wang P, Han Y, Wang X. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics 2012; 75: 1079-88.
7.
Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 2016; 27: 3253-65.
8.
Chen Y, Lu T, Pettersson-Kymmer U, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet 2023; 55: 44-53.
9.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018; 362: k601.
10.
Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res 2019; 4: 186.
11.
Jørgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012; 2: e000990.
12.
Courant F, Antignac JP, Monteau F, Le Bizec B. Metabolomics as a potential new approach for investigating human reproductive disorders. J Proteome Res 2013; 12: 2914-20.
13.
Zhang Z, Zhang Y, Liu C, et al. Serum metabolomic profiling identifies characterization of non-obstructive azoospermic men. Int J Mol Sci 2017; 18: 238.
14.
Zhang J, Huang Z, Chen M, et al. Urinary metabolome identifies signatures of oligozoospermic infertile men. Fertil Steril 2014; 102: 44-53.
15.
Alipour H, Duus RK, Wimmer R, et al. Seminal plasma metabolomics profiles following long (4-7 days) and short (2 h) sexual abstinence periods. Eur J Obstet Gynecol Reprod Biol 2021; 264: 178-83.
16.
Yang J, Zong X, Wu G, Lin S, al Feng Y, Hu J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids 2015; 47: 1549-58.
17.
Malivindi R, Santoro M, De Rose D, et al. Activated-farnesoid X receptor (FXR) expressed in human sperm alters its fertilising ability. Reproduction 2018; 156: 249-59.
18.
Chang D, Li F, Kang Y, et al. The effects of L-carnitine and fructose in improved Ham’s F10 on sperm culture in idiopathic severe asthenospermia within 24h. PLoS One 2025; 20: e0306235.
19.
Peña FJ, Ortiz-Rodríguez JM, Gaitskell-Phillips GL, Gil MC, Ortega-Ferrusola C, Martín-Cano FE. An integrated overview on the regulation of sperm metabolism (glycolysis-Krebs cycle-oxidative phosphorylation). Anim Reprod Sci 2022; 246: 106805.
20.
Eirefelt S, Stahlhut M, Svitacheva N, et al. Characterization of a novel non-steroidal glucocorticoid receptor agonist optimized for topical treatment. Sci Rep 2022; 12: 1501.
21.
Wittmann A, Grimm MOW, Scherthan H, et al. Sphingomyelin synthase 1 is essential for male fertility in mice. PLoS One 2016; 11: e0164298.
22.
Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016; 2: 16057.
23.
Ding X, Deng Y, Wang Y, et al. Serum metabolomic profiling reveals potential biomarkers in assessing the management of women with polycystic ovary syndrome: a randomized controlled trial. Chin Med J (Engl) 2022; 135: 79-85.
24.
Morel Y, Roucher F, Plotton I, Goursaud C, Tardy V, Mallet D. Evolution of steroids during pregnancy: maternal, placental and fetal synthesis. Ann Endocrinol (Paris) 2016; 77: 82-9.
25.
Liu Y, Zhai J, Chen J, Wang X, Wen T. PGC-1 protects against oxidized low-density lipoprotein and luteinizing hormone-induced granulosa cells injury through ROS-p38 pathway. Hum Cell 2019; 32: 285-96.
26.
Jansen H, Lieb W, Schunkert H. Mendelian randomization for the identification of causal pathways in atherosclerotic vascular disease. Cardiovasc Drugs Ther 2016; 30: 41-9.
27.
Mangione R, Pallisco R, Bilotta G, et al. Bilirubin concentration in follicular fluid is increased in infertile females, correlates with decreased antioxidant levels and increased nitric oxide metabolites, and negatively affects outcome measures of in vitro fertilization. Int J Mol Sci 2023; 24: 10707.
28.
Hood RB, Liang D, Tan Y, et al. Serum and follicular fluid metabolome and markers of ovarian stimulation. Hum Reprod 2023; 38: 2196-207.
29.
Li J, Zhang Z, Wei Y, Zhu P, al Yin T, Wan Q. Metabonomic analysis of follicular fluid in patients with diminished ovarian reserve. Front Endocrinol (Lausanne) 2023; 14: 1132621.
30.
Smith LP, Nierstenhoefer M, Yoo SW, Penzias AS, Tobiasch E, Usheva A. The bile acid synthesis pathway is present and functional in the human ovary. PLoS One 2009; 4: e7333.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.