Introduction
Over the past few decades, breast cancer (BC) has become the most often reported disease globally, surpassing even lung cancer in terms of incidence. In 2020, 2.3 million women were diagnosed with breast cancer. By 2040, more than 3 million new cases are expected [1].
Breast cancer is the most frequent tumour and the main cause of cancer-related deaths among women globally [2]. Despite advances in breast cancer therapies that have reduced the chance of metastasis and increased women’s survival rates, a significant percentage of breast cancer survivors are forced to live with long-term issues such as breast cancer-related lymphoedema [3].
For cancer treatment, surgery, chemotherapy, radiation, and hormonal therapy are frequently used. These therapies have a wide range of physical, mental, and psychological consequences such as sleep and mental disorders, anxiety, pain, musculoskeletal dysfunction, and impaired heart and lung function [4].
As a result of BC treatments, lymph vessels may be damaged, impairing lymph transport capacity and resulting in the build-up of protein-rich fluid in the body. It is estimated that up to 1 in 5 women who have breast cancer develop lymphoedema as a result of their treatment [3]. Post-mastectomy lymphoedema (breast cancer-related lymphedema – BCRL) often occurs and compromises physical, psychological, and mental health [5].
Women with BCRL suffer from more pain, upper limb oedema, reduced shoulder mobility, muscle weakness, and poor quality of life [6, 7].
Recently, photo-bio modulation therapy (PBMT) or low-level laser therapy (LLLT) has been widely used in the treatment of BCRL patients. It has been proved that non-invasive phototherapy LLLT improves lymphatic function, stimulates lymphangiogenesis, reduces inflammation, softens fibrous tissues, and alleviates pain [8].
A review by Lima et al. proved the efficacy of low-laser therapy in the management of lymphoedema, but they were unable to perform a meta-analysis because they faced several restrictions such as methodological limitations of the selected studies and a lack of details regarding LLLT [9].
An additional review by Baxter et al. found that LLLT might be a useful therapy strategy for female BCRL. However, their results were not robust due to the limited number of clinical trials included and a lack of formal methodology [10].
Despite these advancements, there remains a notable gap in the literature concerning the use of LLLT in treating BCRL. This review addresses this gap by investigating the effect of LLLT on arm circumference, volume, hand grip, pain, shoulder ROM, and quality of life of patients with BCRL.
Material and methods
This protocol has been registered with the PROSPERO registry (CRD42023468038). PRISMA guidelines were followed when designing the methodology for this systematic review and meta-analysis.
Inclusion and exclusion criteria
The criteria for inclusion were as follows: (1) randomised controlled trials (RCTs); (2) BCRL women; (3) LLLT was the intervention of interest as a single therapy or in combination with any other standard treatment; (4) the outcomes should include at least one of the following: arm circumference, volume, shoulder range of motion, pain, or quality of life (QOL); (5) articles published in English language.
The exclusion criteria were studies based on animal data, non-English language studies, non-breast cancer lymphoedema, and research designs other than RCTs, such as observational studies, reviews, guidelines, surveys, commentaries, and editorial letters.
Information sources
The following databases were electronically searched using the following keywords: Scopus, PEDro, PubMed, Cochrane, and Web of Science. The following keywords were used: low-level laser OR laser therapy OR photo-bio modulation, breast cancer OR post-mastectomy OR lymphedema. Articles published between 2010 and August 2023 were searched. Rayyan QCRI software was utilised to conduct the eligibility screening, as well as to improve collaboration among reviewers during eligibility assessment [11].
Search strategy
Three independent authors evaluated the selected papers against titles and abstracts. Those deemed promising were further subjected to a detailed full-article assessment. Further manual screening was carried out to obtain the associated studies’ reference list.
Selection process
The selected publications were evaluated using Rayyan software, which was refined to eliminate duplicates and then compared to the inclusion criteria. This process was carried out independently by 2 authors. To be sure, the entire article was downloaded and examined for any incomplete or missing information. A third reviewer’s perspective resolved any disputes between the reviewers.
To filter the characteristics of eligible studies, the team developed a characteristics table using a Microsoft Excel 2016 spreadsheet, which included the author’s name, publication year, study design, inclusion criteria, interventions, laser device/parameter, outcomes measurement, follow-up, and conclusion.
Quality assessment
The quality of the included studies was assessed by 2 authors using the PEDro scale, and any conflicts were settled by a third author. PEDro is a validated instrument designed to evaluate the quality of RCTs, and it was widely used in previous systematic reviews [12, 13]. A PEDro score below 4 indicates poor quality, a score between 4 and 5 is fair, a score between 6 and 8 is acceptable, and a score between 9 and 10 is excellent quality [14].
We used the modified Sackett scale to interpret the results and decide the evidence level for each trial [15, 16].
Statistical analysis
Review Manager software, version 5.4, was used for all statistical analyses. Meta-analysis was performed only if there were sufficient clinical and methodological similarities among the included studies. A weighted mean difference (MD) or standardised mean difference (SMD) was calculated to estimate the impact size of continuous outcomes, at a 95% confidence interval, after the heterogeneity was evaluated using the I2 test [17].
Results
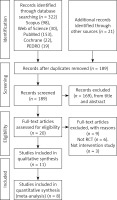
Based on the initial search, we identified 324 studies: Scopus (n = 98), Web of Science (n = 30), PubMed (n = 153), Cochrane (n = 22), PEDro (n = 19); more records found through other sources (n = 2). Eleven studies were eventually accepted for review after the papers were screened for eligibility, as shown in Figure 1 [18–28].
These 11 trials involved 379 female participants, all of whom were diagnosed with BCRL and aged ≥ 18 years. Details regarding sample characterisation, interventions, laser device/parameter, follow-up, main outcomes, follow-up period, and study conclusion are illustrated in Supplementary Table SI.
The PEDro scale rating of the selected studies was as follows: 5 studies were fair (4–5) with a limited level of evidence [19–21, 24, 26], and 6 studies were good (6–7) with moderate evidence 1b [18, 22, 23, 25, 27, 28].
Meta-analysis
The selected trials’ PEDro scale scores ranged between fair [19–21, 24, 26] and good [18, 22, 23, 25, 27, 28]. Three trials were not included in the meta-analysis because the required data were missing [21, 27, 28] (Supplementary Table SII).
Limb circumference
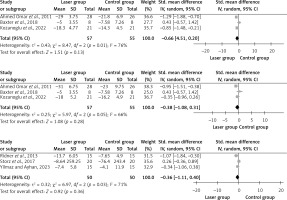
Four studies examined the difference in terms of reduction of limb circumference between affected and unaffected arms [19, 22, 25, 27], but only 3 of them were pooled [19, 22, 25]. These 3 studies assessed the after-treatment effect, as well as follow-up.
The I2 value showed moderate to high heterogeneity among trials, ranging from 66% to 76% at different times. Three months post-intervention, the pooled mean difference was –0.66 (95% CI: –1.51, 0.20) with an overall impact of Z = 1.51, p = 0.13. After the therapy, it was –0.38 (95% CI: –1.08, 0.31) with an overall effect of Z = 1.08, p = 0.28. Following LLLT therapy, there was no significant variation in limb circumference across the groups (Figure 2).
Arm volume
Eight studies examined the difference in terms of arm volume reduction between both arms (affected and non-affected) [18, 20, 21, 23, 24, 26–28], but only 3 studies were pooled [18, 23, 24]. The mean difference in the limb circumference was –0.36 (95% CI: –1.11, 0.40) with an overall effect of Z = 0.92, p = 0.36. There was no significant difference between both groups in terms of arm volume after LLLT. The I2 value was 71%, indicating a moderate level of heterogeneity among the studies (Figure 2).
The results reported by Bramlett et al. (2014), Khalaf et al. (2013), Kilmartin et al. (2020), Mogahed et al. (2020), and Mokhtar et al. (2020) were not pooled because the mean SD was not provided; instead, they just showed the percentage of change.
Hand grip strength
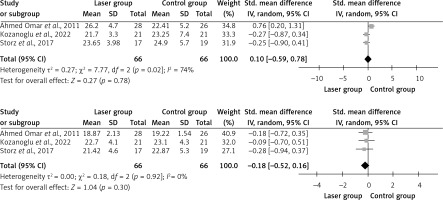
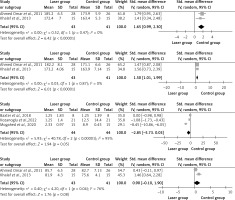
Three studies that examined the hand grip strength [19, 23, 25] were pooled. The mean difference in hand grip was –0.14 (95% CI: –0.59, 0.78) with an overall effect of Z = 0.27, p = 0.78 at 3 months post-intervention, and –0.18 (95% CI: –0.52, 0.16) and with an overall effect of Z = 1.04, p = 0.30 at the end of treatment (Figure 3). Following LLLT, there was no significant variation in hand grip strength across the groups. The I2 value showed low-to-moderate heterogeneity among trials, ranging from 0 to 74% at different times.
Pain score
Pain intensity was studied in 7 of the RCTs [19, 20, 22–24, 27, 28]. All the selected studies showed significant improvement in pain intensity. However, only 3 studies were pooled [19, 20, 22], of which 2 used VAS [19, 20] and one used NRS [22]. The pooled mean difference in pain was –2.85 (95% CI: –5.73, 0.03) with an overall effect of Z = 1.94, p = 0.05. There was a significant difference between the groups in terms of pain intensity after treatment. The I2 value was 95%, indicating high heterogeneity among the studies (Figure 4).
Shoulder mobility
Three clinical studies examined shoulder ROM [25, 26, 28], of which 2 were pooled [25, 26]. It was found that using LLLT for BCRL improved the patient’s shoulder flexion and abduction after LLLT. However, the study by Ahmed Omar et al. mentioned that there were no effects on external rotation [25]. On the other hand, a trial by Khalaf et al. showed improvement in external rotation [26].
The pooled mean difference in shoulder flexion post-treatment was 1.5 (95% CI: 1.01, 1.99) with an overall effect of Z = 6.01, p < 0.00001. There was a positive effect on both groups in shoulder flexion post-treatment. The I2 value was 0%, indicating no heterogeneity among the studies.
The mean difference in shoulder abduction post-treatment was 1.65 (95% CI: 0.99, 2.3) with an overall effect of Z = 6.42, p < 0.00001. There was a significant positive effect between the groups in terms of shoulder abduction after the treatment. The I2 value was 0%, indicating no heterogeneity among studies.
The mean difference in shoulder external rotation post-treatment was 0.75 (95% CI: –0.10, 1.9) with an overall effect of Z = 1.76, p = 0.04. There were significant positive effects between the groups in terms of shoulder external rotation post-treatment. The I2 value was 76%, indicating high heterogeneity among the studies (Figure 4).
Quality of life
Life quality was evaluated in 4 studies [18, 21, 23, 24]. Since they simply provided the percentage of change rather than the mean SD, none of them were pooled. Furthermore, they used different scales, i.e. 2 trials used the upper limb lymphedema-27 (ULL-27) [21, 24], and one trial done by Yilmaz and Ayhan (2023) used LYM-QoL-Arm. In addition, the fourth clinical trial done by Storz et al. (2017) utilised 2 different questionnaires: the McGill Quality of Life Questionnaire (MQOL) and the German version of the Multidimensional Mood State Questionnaire (MMSQ).
The results of the trial done by Mokhtar et al. in 2020 showed positive effects in the social and physical domains and no changes in the psychological domain. Other studies done by Ridner et al. (2013), Yilmaz and Ayhan (2023), and Storz et al. (2017) showed little change throughout the study without any differences between the groups.
Discussion
Low-level laser therapy (LLLT) is used to treat lymphoedema, as well as musculoskeletal problems and other diseases. Furthermore, in clinical settings, patient satisfaction with therapy increased. RCT results have shown that laser therapy is not only beneficial but also time-efficient in treating post-mastectomy lymphoedema when compared to traditional therapies [29].
The current review was conducted to determine the impact of LLLT in the treatment of BCRL. Based on the PEDro scale, the current review provided good to fair evidence (6 trials were good and 5 trials were fair). The meta-analysis of the included trials revealed no significant effects between both groups (laser and control) on arm circumference, arm volume, hand grip strength, and life quality post-intervention, and the study groups differed significantly in terms of shoulder mobility and pain severity. Heterogeneity was evaluated using the I2 test; it was 95% for pain, indicating a high level of heterogeneity among studies, and moderate to high heterogeneity for limb circumference, which ranged from 66% to 76% at different treatment times; and 71% for arm volume means moderate heterogeneity; regarding the hand grip, it showed moderate heterogeneity, i.e. it was 74% at 3 months post-treatment and then became homogenous 0% after the final assessment post-intervention. The I2 value was 0% for shoulder flexion and abduction, indicating no heterogeneity among the studies. However, for shoulder external rotation, it was 76%, indicating high heterogeneity among studies.
Low-level laser therapy (LLLT) has been in use globally since 1995 and received FDA approval in 2007. LLLT helps to increase lymphatic contractility by promoting lymphangiogenesis and lymphatic motility, relaxing fibrotic tissues, and increasing macrophage activity. This permits the fluid to reach the extracellular space [24, 30, 31].
The findings reported by both Lima et al. and Omar et al. showed favourable results in agreement with that of the current review in lowering limb volume and reducing tissue hardness [9, 32].
A meta-analysis by Smoot et al. demonstrated that LLLT was clinically beneficial in the management of BCRL because there was evidence of clinically relevant pain and volume reductions after the completion of LLLT treatments within the groups [5]. Furthermore, a systematic review by Baxter et al. showed the efficacy of LLLT in decreasing the volume and circumference of the affected arm, as well as pain reduction greater than placebo therapy [10].
On the other hand, the systematic review done by Ywang in 2022 established that LLLT did not substantially enhance the evaluated outcomes (limb circumference, volume, hand grip, and pain) as compared to compression therapy, placebo laser, or no treatment at all for BCRL patients [31]. Inconsistent results might have been caused by the wide variability in laser treatment regimens (wavelength, dose, duration, frequency, and emitting zone). The impact of various laser treatment parameters on biological regulation may differ. The biological control of laser treatment is contingent upon the chromophore’s ability to absorb light. Only photons falling inside a particular wavelength range are absorbed by each chromophore. However, the degree of the cellular action varies depending on the quantity of energy provided, even with a suitable wavelength [33].
According to a systematic review by Mahmood et al., women with lymphoedema secondary to breast cancer reported improvements in their arm volume and circumference but not in their shoulder range of motion and pain [34].
In the current review, there was a remarkable improvement in shoulder flexion, as well as abduction and external rotation post-treatment, with p-values of < 0.00001 and < 0.00001, respectively. The results of a trial by et al. showed that low-level laser therapy did not improve shoulder mobility in BCRL patients [28].
In breast cancer survivors, BCRL can negatively impact their life quality by decreasing their physical function and increasing their disability [35, 36]. The trials included in the current review showed little change throughout the study periods, and no significant differences between the study groups were observed in LLLT for BCRL.
The underlying mechanism of benefit of using LLLT as a therapeutic intervention for BCRL patients is that light absorbs energy and increases the synthesis of metabolic energy and oxygen consumption, both of which are necessary for cell repair, by activating cytochrome oxidase in the mitochondrial membrane. Research on the application of low-intensity lasers indicates that they stimulate lymphocytes, local fluid circulation, macrophages, and the immune system, thereby reducing infection risks [37, 38].
LLLT has been demonstrated to have anti-inflammatory and anti-oedematous properties by raising prostaglandin I2, which inhibits platelet aggregation and vasodilation, resulting in oedema reduction and better tissue oxygenation. There are a few reasons why lymphoedema following mastectomy may benefit from LLLT: (1) it aids in the resorption of oedema fluids, both large and small; (2) it promotes regeneration, contractility, and diameter of lymphatic vessels; (3) it promotes neutrophil and monocyte phagocytosis; (4) it reduces scar adhesion and improves wound healing; and (5) it decreases the risk of skin infections [25, 39].
To minimise bias, our systematic review carefully adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 (PRISMA) standards. Second, Sackett’s level of evidence was used to analyse the research based on the methodological quality and validity of its design. Finally, the findings of the review were gathered from 11 fair- to good-quality studies.
Our study limitations were that the current work included only trials that were published in scientific journals, and other published materials in conferences were not considered, which may affect the results. Secondly, non-English publications were not included. Moreover, none of the analysed studies were of high quality because only fair to good-quality studies were available.
In conclusion, although LLLT (PBM) was effective in reducing arm circumference, volume, and pain, as well as improving grip strength, life quality, and mobility, the meta-analysis did not show significant improvement except in pain and shoulder mobility. Further research is required to determine the long-term safety and effectiveness of PBMT in the treatment of BCRL. The clinical implication of our review is that low-level laser therapy might be effective in reducing lymphoedema volume and pain, as well as enhancing hand grip strength, quality of life, and mobility, along with significant improvement in pain and shoulder movement.