Introduction
Critically ill patients often require respiratory treatment with a ventilator, but prolonged use of a ventilator may lead to ventilator-associated pneumonia (VAP). Ventilator-associated pneumonia is a leading cause of death in critically ill patients, with a mortality rate of 30–50% [1]. Common pathogenic bacteria associated with VAP include Staphylococcus aureus, Streptococcus pneumoniae, Acinetobacter baumannii and Pseudoquas aeruginosa. Despite the efficacy of antibiotics in combatting bacterial infections, their extensive use has contributed to the emergence of progressively more drug-resistant strains, posing great challenges to the clinical treatment of ventilator-associated pneumonia [2]. Tigecycline is a glycyclin with superior antibacterial properties against Gram-negative bacilli, positive bacilli, and anaerobic bacteria [3]. However, with the increasing clinical application of tigecycline, many researchers have observed that the recommended dosage of tigecycline may be inadequate for the treatment of ventilator-related pneumonia caused by multi-resistant bacteria [4]. Further investigation into drug-resistant strains has revealed that high-dose tigecycline can significantly improve the clearance rate of multi-resistant bacteria and improve the treatment effect of ventilator-related pneumonia [5]. Accordingly, a thorough review of literature databases was conducted to investigate various doses of tigecycline for treating ventilator-associated pneumonia caused by multidrug-resistant bacteria. A subsequent meta-analysis was performed to evaluate the efficacy and safety of high-dose tigecycline treatment for this condition. The results of this study aim to provide a theoretical foundation for the clinical management of patients with multidrug-resistant ventilator-associated pneumonia, and are presented as follows.
Material and methods
Literature search strategy
A systematic search was conducted in the Cochrane Library, Embase, PubMed, Chinese biomedical literature database, CNKI, literature retrieval in Chinese journal full text database, Wanfang, and web-based databases using a combination of keywords and free text. The Chinese search terms included “teacycline, different doses, ventilator-associated pneumonia”, while the English search terms were “Tigacycline, Drug-resistant bacteria, and Ventilator-associated pneumonia”. The search period for database construction was from inception until December 2022, and the search was conducted in both Chinese and English languages. The registration number is INPLASY 202340034. The DOI number is 10.37766/inplasy2023.4.0034.
Literature inclusion and exclusion criteria
Inclusion criteria: 1. All the included studies are randomized controlled experiments. 2. The study subjects were patients with ventilator-associated pneumonia caused by multi-drug-resistant bacteria, and the indicators reflecting the clinical effect and safety were taken as the observation indicators (such as treatment effect, adverse effects, etc.). 3. The treatments involved include different doses of tigecycline, placebo, etc.
Exclusion criteria: 1. Non-randomized controlled study. 2. Incomplete data in the article and repeated published literature. 3. Animal experiments, case reports, reviews, etc.
Data extraction and literature quality evaluation
The evaluation of literature quality was conducted using the literature evaluation criteria of the Cochrane System Evaluator Manual and the modified Jadad scale. In instances where there were divergent opinions, a third researcher was consulted to research a consensus. The modified Jadad scale score was based on randomized sequence generation, randomized concealment, blindness, and withdrawal criteria, with a score range of 1–3 representing low quality and 4–7 indicating high quality.
Statistical analysis
Statistical analysis was performed using Stata software. The included literature underwent heterogeneity testing using Cochran’s Q test, with a statistical I2 representation of > 50% or p < 0.05 indicating statistical heterogeneity among the literature. In the presence of statistical heterogeneity, the random effect model incorporated the effect size; conversely, the fixed effect model was employed. The weighted mean difference (WMD) and 95% confidence interval (95% CI) were used to represent the combined effect size of measurement data. RR and 95% CI were used for interval assessment of the combined effect size of counting data. Publication bias was assessed using a funnel plot and Egger’s test, with the test level set at α = 0.05.
Results
Basic characteristics of included literature
The initial step involved searching relevant databases for keywords related to the study title, followed by reviewing the abstracts of identified literature. Articles meeting the inclusion criteria were downloaded, and their content was thoroughly assessed against the exclusion criteria. Ultimately, a total of 74 Chinese articles and 30 English articles were retrieved, and after careful examination, six articles were considered for analysis [6–11]. The literature screening flow chart is shown in Figure 1. The six included articles comprised 509 patients, with 280 in the control group and 229 in the observation group. The treatments employed in the studies included tigecycline and meropenem.
Basic characteristics of the literature included in Table I.
Table I
Basic characteristics
Meta-analysis results
Clinical efficacy
Clinical efficacy of different doses of tigecycline in patients with ventilator-associated pneumonia caused by multiple drug-resistant bacteria
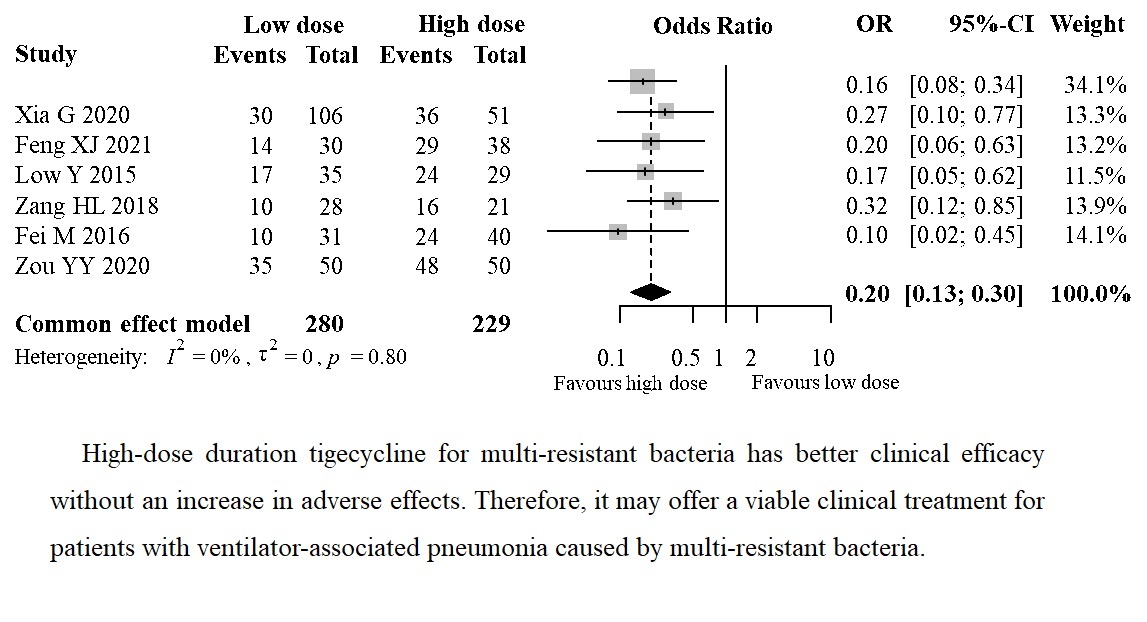
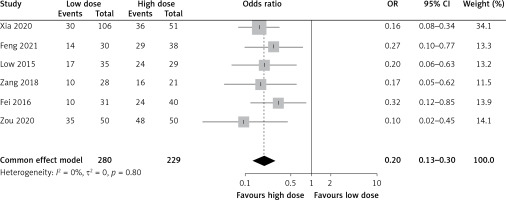
The analysis included six articles that reported the clinical efficacy of different doses of tigecycline in patients with ventilator-associated pneumonia caused by multiple resistant bacteria. The meta-analysis revealed no clinical or statistical heterogeneity between studies (I2 = 0%, p = 0.80), and a fixed-effect model was used to determine the effect size. The clinical effect of the observation group was significantly better than that of the control group (OR = 0.20, 95% CI: 0.13–0.30, p < 0.00001), as shown in Figure 2.
Publication bias
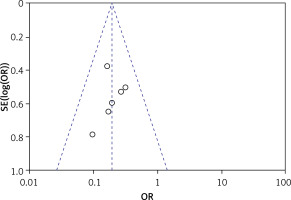
Using a funnel plot and Egger’s test, none of the six articles with clinical efficacy as an effect index were statistically significant (p > 0.05), suggesting no publication bias, as shown in Figure 3.
Adverse reactions
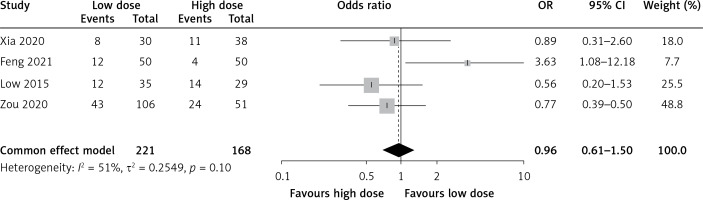
Adverse effects of different doses of tigecycline treatment in patients with ventilator-associated pneumonia caused by multiple drug-resistant bacteria
A total of 6 articles reported the adverse effects of tigecycline in patients with ventilator-associated pneumonia caused by different doses of multiple resistant bacteria. The meta-analysis revealed no clinical heterogeneity or statistical heterogeneity between the included studies (I2 = 51%, p = 0.10). The effect size was incorporated using a fixed-effect model, indicating that the incidence of adverse effects in the observed group was similar to that of the control group (OR = 0.96, 95% CI: 0.61–1.50, p = 0.85), as shown in Figure 4.
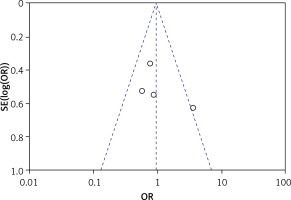
Publication bias
Using a funnel plot and Egger’s test, none of the four articles with adverse effects as effect indicator were statistically significant (p > 0.05), suggesting no publication bias, as shown in Figure 5.
Discussion
Ventilator-related pneumonia is a prevalent clinical condition that usually occurs in respiratory critical care units. Reports indicate that gland-negative bacteria account for a significant proportion of these cases, with the trend increasing annually. Additionally, with the extensive use of clinical antibiotics, the prevalence of multi-drug-resistant strains is also on the rise. Therefore, the treatment of ventilator-associated pneumonia resulting from multi-drug-resistant bacteria has become a challenging issue in clinical practice.
Tigecycline is an antibiotic that exhibits a broad antibacterial spectrum and has been shown to be effective against multiple resistant bacteria. However, the conventional doses of tigecycline (maintenance dose at 50 mg, once every 12 h) have limited efficacy in treating ventilator-related pneumonia caused by multi-resistant bacteria. This is due to the low strain clearance rate and extended drug use time and hospitalization duration. On the other hand, high-dose tigecycline (maintenance dose at 100 mg, once every 12 h) has demonstrated certain advantages over conventional doses in treating multiresistant bacteria. Nevertheless, clinical scholars are concerned whether high-dose tigecycline treatment would cause more serious adverse reactions. Therefore, to further understand the treatment efficacy and safety of different doses of tigecycline in treating patients with multi-resistant bacteria, a meta-analysis was conducted. Six articles, including five in Chinese, were selected according to the inclusion and exclusion criteria. The meta-analysis revealed that high-dose tigecycline was significantly more effective than the control group in treating patients with multi-resistant pneumonia (OR = 0.20, 95% CI: 0.13–0.30, p < 0.00001). The results suggest that high doses of tigecycline in patients with multi-resistant bacteria are more effective, and can effectively improve the therapeutic effect. Safety is also a key concern for clinicians, and the meta-analysis showed that the incidence of adverse reactions in the observed group did not differ from the control group (OR = 0.96, 95% CI: 0.61–1.50, p = 0.85). The results suggest that high doses of tigecycline do not increase the incidence of adverse effects.
The study has the following limitations. The lack of specific method descriptions regarding random sequence generation, no mention of allocation concealment, and no double-blinding in the included studies may lead to bias in selection, implementation, and detection, and can lead to false-positive results. Second, the included studies were all single-center studies, with small sample sizes and varying lengths of interventions, which may lead to large differences in results. Third, this study only compared the differences between low-dose and high-dose tigecycline treatment, but the dose was inconsistent between different studies [12–20].
In conclusion, high-dose tigecycline for multi-resistant bacteria has better clinical efficacy without an increase in adverse effects. Therefore, it may offer a viable clinical treatment for patients with ventilator-associated pneumonia caused by multi-resistant bacteria.