Breast cancer (BC) is one of the most common malignancies worldwide in women (2.3 million new cases occurred in 2020) [1]. Approximately 40% (range: 35–50%) of newly diagnosed patients with BC are women ≥ 65 years old. In certain studies, ≥ 65 years of age has been reported as a negative prognostic factor in BC [2, 3]. The National Comprehensive Cancer Network St. Gallen and European Society of Medical Oncology guidelines have proposed that patient age should be considered as a prognostic factor [4, 5]. BC in elderly women is supposed to have less aggressive biology, as indicated by a higher rate of hormone-receptor-positive tumors [6], lower grading and lower proliferation rates compared with those of younger patients [7]. However, tumor stage at primary diagnosis is commonly more advanced [8]. The objective of the present study was to characterize > 65-year-old patients according to clinicopathological and molecular factors (BRCA, checkpoint kinase 2 (CHEK2) and nucleotide binding oligomerization domain containing 2 (NOD2) mutation), as well as blood platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) for their prognostic value. The present study also compared 65-year-old patients with < 50-year-old patients. Overall survival (OS) analysis was performed for both groups, separately and combined.
Methods
A total of 723 female patients with BC who had been diagnosed and treated at the National Research Institute of Oncology, Gliwice Branch, Poland, during 2005–2019, were retrospectively reviewed in the present study. Two groups of patients were distinguished: women with BC > 65 years of age (92 patients) and control patients < 50 years of age (306 patients). We analyzed 398 women from all 723 patients. Table I shows the clinicopathological characteristics of patients in the subgroups > 65 and < 50 years of age. In the present study a retrospective analysis was conducted on medical records and results of laboratory tests. All patients provided written informed consent regarding the use of their biological material and data for clinical research (of note, all the tests conducted were routine laboratory analyses). The prognostic value regarding OS of various laboratory parameters, including PLR, NLR and MLR, was assessed based on univariate analysis. Optimal cut-off values for NLR, PLR and MLR were determined using receiver operating characteristic curve analysis. The maximum value of Youden’s index was used as a criterion for selecting the approximate cut-off value of laboratory parameters. Based on the determined cut-off values, NLR > 1.88 was considered ‘elevated’; MLR > 0.27 was considered ‘elevated’; and PLR > 134.20 was considered ‘elevated’.
Table I
Clinicopathological characteristics of patients
The status of CHEK2*1100delC and I157T mutations (GenBank NM_007194.3) was assessed using allele-specific amplification PCR and restriction fragment length polymorphism PCR techniques. The present study examined the most common mutations in BRCA1 (c.68_69delAG, c.181T>G, c.4034delA, c.5266dupC and c.3700_3704del5; GenBank NM_007294.3) and BRCA2 (c.5946delT and c.9403delC; GenBank NM_000059.3) present in the Silesian population. The presence of the c.3016_3017insC mutation of NOD2 (GenBank NM_022162.1) was also evaluated in the whole study group.
Statistical analysis
Statistical analyses were performed using Dell Statistica 13 software. Qualitative factors were presented as numbers and percentages. Comparisons between patient subgroups were performed with Fisher’s exact test and the χ2 test with Yates’ correction. The results of laboratory parameters in the subgroups of patients aged > 65 and < 50 years were expressed as median values with interquartile ranges. The Mann-Whitney U test was used to compare the two subgroups. OS was estimated using the Kaplan-Meier method and was compared using the log-rank test. Cox proportional hazard regression for univariate and multivariate analyses of prognostic factors was applied. Factors with p < 0.10 in univariable Cox analysis were used in multivariable Cox analysis. P < 0.05 was considered to indicate a statistically significant difference.
Results
Histological grade G3 was observed significantly more frequently in the group of patients of < 50 years of age compared with the findings in the group of elderly patients (> 65 years of age) (39.2 vs. 25.0%; p = 0.013). Positive steroid receptor status (estrogen receptor (ER+)/progesterone receptor (PR+)) was observed more frequently in patients > 65 years of age, although the results were not statistically significant (76.1 vs. 66.3%; p = 0.096). The triple negative BC subtype was more common in < 50-year-old patients in comparison with the findings in > 65-year-old patients (21.9 vs. 13.0%; p = 0.073). By contrast, the luminal A BC subtype was observed more frequently in the group of 65-year-old patients than in younger women (26.1 vs. 15.0%; p = 0.019) (Table I).
In patients aged > 65 years, higher values of lymphocytes (median: 2.01; interquartile range: 1.80–2.41 vs. 1.82 (1.49–2.17); p = 0.001) and lower PLT counts (246 (212–269) vs. 261 (227–302); p = 0.004) were observed in comparison with the findings in the group of patients < 50 years of age. Similarly, in the group of patients > 65 years of age, lower NLR (1.72 (1.34–2.41) vs. 1.93 (1.55–2.66); p = 0.008) and lower PLR (123 (97.4–145.6) vs. 141.4 (115.1–182.5); p = 0.0001) were observed than in younger women (aged < 50 years). Lower MLR values were also observed more in > 65-year-old patients (0.24 (0.20–0.31) vs. 0.28 (0.21–0.33); p = 0.070).
The presence of mutations (BRCA, CHEK2 or NOD2) in patients compared with the control group was also compared. BRCA mutations were detected significantly more often in patients aged < 50 years in comparison with the findings in women aged > 65 years (19.0 vs. 7.1%; p = 0.024). Similarly, CHEK2 mutations were more often detected in younger patients, although the results were not significant (10.9 vs. 4.4%; p = 0.147). No association was detected between the presence of NOD2 mutations and patient’s age (26.0 vs. 22.6%; p = 0.563).
Univariate analysis of clinical and pathological factors, such as tumor size (T), lymph node status (N), tumor grade (G), ER status and HER2 overexpression, in patients aged > 65 years showed that only N (N+ vs. N0; hazard ratio (HR = 2.78); p = 0.025) and G (G3 vs. G1-2; HR = 2.69; p = 0.031) were significant factors. Similarly, in multivariate analysis, lymph node status (HR = 2.98; p = 0.017) and tumor grade (HR = 2.92; p = 0.020) were significant prognostic factors. By contrast, in the subgroup of patients aged < 50 years, univariate analysis showed that tumor size (T3-4 vs.T1-2; HR = 3.38; p = 0.0001), lymph node status (N+ vs. N0; HR = 1.86; p = 0.039), ER status (ER+ vs. ER-; HR = 0.40; p = 0.003) and HER2 overexpression (HER2+ vs. HER2-; HR = 1.90; p = 0.032) were significant prognostic factors. In multivariate analysis, only tumor size (HR = 2.84; p = 0.001) and ER status (HR = 0.45; p = 0.010) were significant prognostic factors associated with OS. Laboratory parameters such as NLR (HR = 0.897; p = 0.670), MLR (HR = 0.998; p = 0.992) and PLR (HR = 0.957; p = 0.866) were not significantly associated with OS in univariate analysis.
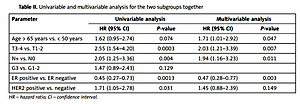
Table II shows the results of univariate and multivariate analysis of the two subgroups of patients together. In the univariate analysis, tumor size (HR = 2.55; p = 0.0003), lymph node status (HR = 2.05; p = 0.004), ER status (HR = 0.45; p = 0.0013) and HER2 overexpression (HR = 1.71; p = 0.031) had a significant impact on OS. Patients > 65 years of age had a worse OS than younger patients, although the difference was not significant (log-rank test p = 0.079). The 5-year OS was 88.1% for younger patients (< 50 years old) and 82.7% for patients aged > 65 years. Multivariate analysis showed that age, T, N and ER were independent prognostic factors. After adjusting for clinical and pathological factors, age > 65 years was observed to be a significant factor for worse OS in comparison with age < 50 years (HR = 1.71; p = 0.047). Higher T (T3-T4) (HR = 2.03; p = 0.007) and presence of lymph node metastases (N+; HR = 1.94; p = 0.007) had a negative impact on OS, while positive ER status (HR = 0.47; p = 0.007) was associated with improved OS.
Table II
Univariable and multivariable analysis for the two subgroups together
Discussion
Clinicopathological analyses of patients with BC > 65 years of age have been conducted previously [8, 9]. Previous studies showed that tumors of elderly patients with BC were characterized by an ER and/or PR positive status, and low expression of EGFR, HER2 and Ki67 [9, 10]. Luminal tumors were more frequently found in elderly patients (> 65 years of age), while Erb-B2 receptor tyrosine kinase 2, basal-like and unclassified subtypes were more often found in young patients (< 65 years of age) [11]. In our previous study, a worse prognosis (OS) was observed in patients with BC who had elevated pre-treatment NLR (> 2.65) (albeit not statistically significant) and PLR (> 190.90) (statistically significant) values [12]. Elevated NLR values (> 2.65) were more frequently reported in younger women (median: 47.7 vs. 53.5 years; p = 0.021). However, PLR or MLR values were prognostic factors independent of age.
In our previous study, women carrying BRCA1 mutations were significantly younger than the control group (43 vs. 53 years old). Similarly, CHEK2 carriers were also of younger age, although this finding was not statistically significant. All carriers of mutations were younger than the control group. Namely, patients with a BRCA1 mutation had a median age of 43 years; those with a NOD2 mutation had a median age of 47 years; and those with a CHEK2 mutation had a median age of 50 years [13].
Patients with BC aged > 65 years were characterized by G1-2 and luminal A BC subtype compared with the findings in the group of patients aged < 50 years. Similarly, higher lymphocyte values, and lower PLT, NLR and PLR values, were characteristics of the group of patients aged > 65 years. BRCA mutations were detected significantly more frequently in patients aged < 50 years. Thus, age > 65 years is a negative prognostic factor independent of other factors.