Epidemiology of lipid disorders and atherosclerotic cardiovascular disease
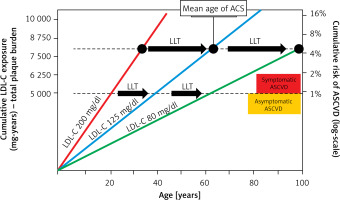
Lipid disorders are the most common modifiable risk factor, and one of the markers most strongly associated with atherosclerotic cardiovascular disease (ASCVD), including coronary artery disease (CAD), ischaemic stroke and peripheral artery disease (PAD) [1]. Cardiovascular disease (CVD), up to two-thirds of which is ASCVD, is the leading cause of death worldwide (Figure 1), making it possible to conclude that these are largely avoidable deaths [2–7]. In 2021, 3.81 million (95% CI: 2.17–5.42) CVD deaths and total deaths were attributed to elevated serum low density lipoprotein cholesterol (LDL-C) levels [8]. Serum LDL-C concentration is the primary lipid parameter used to determine cardiovscular (CV) risk and define the target of lipid-lowering treatment (recommendation class: I, level A), although as of September 2021, according to Polish guidelines from 6 scientific societies, non-high density lipoprotein (non-HDL) cholesterol, which represents the mass of all atherogenic particles, is considered equally important [1]. It should be emphasised that, in the context of ASCVD risk, the duration of exposure to elevated serum LDL-C concentrations is important (Figure 2) [9, 10]. Such a poor epidemiological situation in the context of CVD led the World Health Organisation (WHO) in 2013 to adopt the goal of reducing mortality associated with these diseases by 25% until 2025 [11]. The epidemiological outlook for lipid disorders in Poland is also highly worrying. In our country, the most common lipid disorder is hypercholesterolaemia, which is present in up to three out of four people [1]. The WOBASZ II study (Multicentre National Population Health Survey), which included 5,947 people aged from 20-99, found that hypercholesterolaemia was present in 67.1% of the participants (64.3% of women and 70.3% of men, respectively) [12]. These results indicate that the number of patients with hypercholesterolaemia in Poland may be as high as over 20 million. The epidemiological situation related to hypercholesterolaemia is unfortunately not improving in Poland, as the NATPOL 2011 study, which included 2,412 individuals aged 18–79, found that 61.1% of them had lipid disorders [13]. The number of disability-adjusted life years (DALYs) associated with CVD in Poland is three times higher than in Western European countries, the United States or Australia. The main cardiovascular cause of DALYs in Poland is CAD [2]. The LIPIDOGRAM 2015 study of 13,724 primary care users showed that lipid disorders were present in 83.7% of people without a history of CVD and in 90.8% of patients with CVD [14]. If we add to this the fact that only 24% of people in Poland are on a therapeutic target for LDL-C cholesterol, including only 18% of the highest-risk patients, we can positively state that lipid disorders are the most common, and the worst monitored cardiovascular risk factor in Poland [15]. All this is a strong signal to intensify efforts to diagnose and treat lipid disorders more quickly and effectively, and thus to effectively prevent ASCVD.
Figure 1
Causes of death worldwide according to the results of the Global Burden of Disease Study 2019. Prepared and modified based on [2–7]
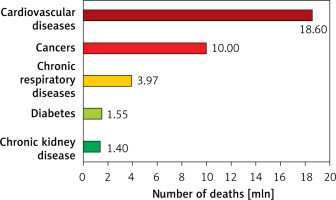
Figure 2
Effect of exposure to different serum LDL-C concentrations on the risk of ACS and the role of LLT in cardiovascular prevention. Redrawn and modified based on Ference BA et al. J Am Coll Cardiol 2018; 72: 1141-56 [10]; CC BY-NC-ND license – no permission required.
LDL-C – low density lipoprotein cholesterol, ACS – acute coronary syndrome, ASCVD – atherosclerotic cardiovascular disease, LLT – lipid-lowering therapy.
Reducing serum LDL-C levels – what are the benefits?
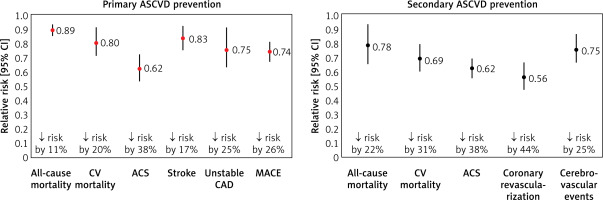
Therapeutic strategies, of which statins are the primary and gold standard, are the mainstay of treatment for patients with ASCVD (Figure 3) [16, 17]. The crucial importance of lipid-lowering treatment in the prevention of ASCVD is evidenced by the fact that each 1% reduction in serum LDL-C concentration is associated with a reduction in CV risk of approximately 1%. After 5 years, that risk is reduced by about 20–25%, and after 40 years by as much as 50–55%, confirming the validity of continuing treatment throughout the patient’s lifetime [18]. A meta-analysis of 21 studies by Wang et al. involving more than 184,000 patients also showed that the longer the lipid-lowering treatment continues, the greater the CV benefit. The authors found that each reduction in serum LDL-C concentration in mmol/l was associated with a 12% (95% CI: 8–16%) reduction in the risk of major CV events (MACE) at year 1, 20% (16–24%) at year 3, 23% (18–27%) at year 5 and 29% (14–42%) at year 7 of lipid-lowering treatment (LLT) [19]. These data point to the need for long-term and consistent (on the part of the patient) reduction of serum LDL-C levels in patients with lipid disorders, because only then will cardiovascular risk be reduced from year to year. Another crucial aspect of lipid-lowering treatment is the question of the intensity of serum LDL-C lowering. A meta-analysis of 26 randomised clinical trials (RCTs) conducted by the Cholesterol Treatment Trialists (CTT) research group, involving a total of 170,000 patients, showed that more intensive lipid-lowering treatment was associated with an additional reduction in the incidence of major vascular events by 15%, CAD death or non-fatal acute coronary syndrome (ACS) by 13%, coronary revascularisation by 19% and ischaemic stroke by 16% [20]. The greater benefit of more intensive LLT is also supported by a meta-analysis of 18 RCTs by Hsu et al., which found that more intensive lowering of serum LDL-C levels was associated with an additional reduction in the risk of CV events by 24% (RR = 0.76; 95% CI: 0.68–0.85) and the risk of death from any cause by 10% (0.90; 0.83–0.97) [21]. The duration of exposure to elevated serum LDL-C concentrations plays a key role in the pathogenesis of ASCVD. A study by Domanski et al. involving 4,958 participants aged 18–30 years followed up for 16 years found that the ASCVD risk was higher in those who were exposed to elevated serum LDL-C concentrations at a younger age, compared to those exposed to elevated LDL-C levels at an older age, highlighting the importance of optimal control of serum LDL-C concentrations from an early age [22]. Furthermore, it is worth mentioning here the results of a study by Vallejo-Vaz et al. involving 3,505 patients with ASCVD, which showed that those with diabetes, chronic kidney disease (CKD) or polyvascular disease (PVD) had greater absolute CV benefits from further reductions in serum LDL-C levels compared with patients without comorbidities [23].
Expert recommendation
Lipid-lowering treatment should be carried out according to the principles “the earlier, the better”, “the lower, the better” and “the longer, the better”, in order to significantly reduce cardiovascular risk and improve prognosis in primary and secondary prevention of ASCVD (IA).
Figure 3
Lipid-lowering treatment with statins in primary and secondary prevention of ASCVD. Based on data from [16, 17]
ACS – acute coronary syndrome, ASCVD – atherosclerotic cardiovascular, CV – cardiovascular, CAD – coronary artery disease, 95% CI – 95% confidence interval, MACE – major adverse cardiovascular events.
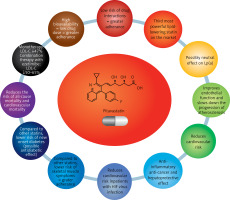
The armamentarium of lipid-lowering treatment – what tools are available?
The gold standard of LLT is statins. Other mainstay medicines for lowering serum LDL-C levels include ezetimibe, proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors/modulators and bempedoic acid [1, 24, 25]. So-called high-intensity/potent statins (atorvastatin, rosuvastatin) are now recommended at maximum tolerated doses, to reduce serum LDL-C concentrations by approximately 50% [26]. More recently, some experts have also added pitavastatin to this group – finally available in most European countries – which, at the highest dose of 4 mg, lowers LDL cholesterol by an average of 43–47% [27, 28]. Depending on the therapeutic target (determined individually for each patient based on CV risk and baseline serum LDL-C concentration), treatment with appropriate lipid-lowering potency is selected [1]. The use of a combination of lipid-lowering drugs allows serum LDL-C concentrations to be reduced by up to ≥ 85% (potent statin at the highest dose + ezetimibe + agents that inhibit the PCSK9 protein, or from 2024 onwards in Poland, additionally in combination with or without bempedoic acid) (Table I) [26]. On this basis, over the past few years, the recommendation of high-intensity statin (HIS) therapy has been replaced with combined high-intensity lipid-lowering therapy, or even combined high-intensity lipid-lowering therapy with HIS and ezetimibe, especially after recent reports suggesting that as many as one in four physicians reduce the statin dose by adding ezetimibe or PCSK9i [29, 30]. In conclusion, the use of lipid-lowering medicines and their combinations can reduce serum LDL-C concentrations by up to ≥ 85%, making it possible to (hypothetically) achieve therapeutic goals in all patients with lipid disorders [31].
Table I
How to ensure the efficacy of lipid-lowering therapy in different groups of patients with very high and extremely high cardiovascular risk requiring high-intensity lipid-lowering therapy. Modified based on [26]
[i] The preferred option for all patients with ASCVD is FDC, which includes intensive therapy with statins and ezetimibe. ASCVD – atherosclerotic cardiovascular disease, LDL-C – low-density lipoprotein cholesterol, ACS – acute coronary syndrome, PAD – peripheral artery disease, HIS – high intensity statin therapy, MIS – moderate intensity statin therapy, FDC – fixed dose combination, EZE – ezetimibe, BA – bempedoic acid, PCSK9m – proprotein convertase subtilisin/kexin 9 modulators – therapy based on the use of proprotein convertase subtilisin/kexin 9 modulators/inhibitors (inclisiran, alirocumab, evolocumab).
Achieving therapeutic goals
In view of the data presented, LLT, mainly using statins, which can significantly prolongs life for most patients in primary and secondary prevention of ASCVD, should be characterised by effective control of serum LDL-C levels. Unfortunately, this is not the case [32, 33]. Despite the availability of excellent lipid-lowering drugs, the possibility to personalise and intensify treatment, and specific treatment guidelines, the control of lipid disorders and the achievement of therapeutic goals is insufficient. Only one in 3 patients in Europe, and one in four in Poland and Central and Eastern Europe, achieves the LDL-C target [34]. The therapeutic target for patients at very high CV risk (i.e. < 55 mg/dl/< 1.4 mmol/l) is achieved in only 18% of the European population, 17% of the Polish population and only 13% in CEE countries [15, 34]. Moreover, the LDL-C target in patients at extreme CV risk (i.e. < 40 mg/dl; < 1 mmol/l) is achieved by less than 10% of patients [15, 34]. When trying to find the most common reasons for this low effectiveness, two aspects seem to be crucial – the use of low, or moderately intensive statin therapy even in > 50% of patients and virtually no combination therapy. A recent SANTORINI study, which included 9,044 patients with high or very high CV risk from 14 European countries, showed only a small improvement, as only 20.1% achieved the target serum LDL-C concentration according to the current 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines (24% of high-risk patients and 18.6% of very high-risk patients, respectively). Furthermore, it was found that as many as 21.8% (!) of patients did not receive any LLT (23.5% and 21.1%), statin monotherapy was used by 54.3% of patients (58.4% and 52.5%), while combination treatment was used in 24% of patients (18.1% and 26.4%) and in most cases involved a combination of statin and ezetimibe [35]. Given the actual prevalence of statin intolerance (which is around 9% or 5–7% assuming diagnosis based on recognised definitions of intolerance [36]), there is no explanation for the > 21% of patients at very high risk of ASCVD and patients with pre-existing ASCVD not taking any lipid-lowering therapy. The CEPHEUS study, which included 33,198 patients from 29 countries in Asia, Western Europe, Eastern Europe, the Middle East, and South Africa, showed that 50.5% of patients achieved target serum LDL-C levels (62.8% and 33.5% in patients treated with primary and secondary ASCVD prevention, respectively). Target serum LDL-C levels were achieved in 74.4%, 57.0% and 25.5% of patients with moderate/moderate-high, high, and very high cardiovascular risk, respectively [37]; on the other hand, this shows at the same time that 3 out of 4 patients with very high risk are still beyond the therapeutic target. A study by Nelson et al. involving 601,934 patients with ASCVD showed that 49.9% were not using statins, only 22.5% were using HIS and 27.6% were using other low- or moderate-intensity statins [38]. A study by Koenig et al., including real-world data of 865,732 patients using statins, 34,490 patients using ezetimibe and 1,940 patients using PCSK9i, found that after 36 months, adherence to therapy remained at 20.6% for statins, 22.3% for ezetimibe and 50.9% for PCSK9i [39] (significantly lower than the ODYSSEY APPRISE and SAFEHEART studies, which showed up to > 97% adherence to therapy [40, 41]). A high rate of non-adherence to therapy was observed in patients with lipid disorders for all lipid-lowering drugs (the worst adherence was with statins and the best adherence was with PCSK9i) [39]. A study by Khachatryan et al. involving 73,275 very high-risk CV patients found that higher adherence and/or intensity of lipid-lowering treatment was associated with a significantly lower risk of CV complications or death from any cause [42]. Another analysis in a group of 347,104 patients with ASCVD showed that the worse the adherence to LLT, the higher the risk of death from any cause, by up to 30% [43].
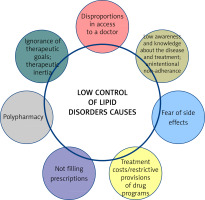
There are many reasons for the poor control of lipid disorders (Figure 4) [44]. Awareness of therapeutic targets in patients with lipid disorders remains a critical factor, hence the importance of knowing the 2019 guidelines, expanded further in 2021, and above all the national guidelines that best reflect the real problems in each country. We find there that not only serum LDL-C, but also non-HDL-C should be assessed, and both are now equivalent elements in the assessment of the lipid profile. Added to this is an independent risk factor which is lipoprotein(a) (Lp(a)), which should be assessed not only in patients with premature CVD or, in the absence of a statin treatment effect, in patients on the borderline between moderate and high CV risk, but also in all patients with ASCVD, with familial hypercholesterolaemia and in pregnant women [1, 45, 46]. In addition to therapeutic inertia, another important aspect is the fear of the side effects of statins, even though they are among the most effective and best-tolerated medicines used in cardiology [47]. Mention should also be made of the availability of medicines to patients, and the overly restrictive reimbursement criteria and prices of some medicines, as well as the almost total lack of education. Indeed, many studies and clinical experience clearly indicate that an educated patient is a patient who is more likely to adhere to treatment and better co-operates with the doctor [48, 49].
In conclusion, despite the availability of effective and safe lipid-lowering drugs, the majority of patients at high, very high and extremely high CV risk do not achieve the therapeutic target for serum LDL-C levels, mainly due to low adherence, therapeutic inertia and lack of proper patient education on the impact of lipid disorders on health.
Expert recommendation
The use of lipid-lowering drugs and their combinations can reduce serum LDL-C concentrations by up to > 85%. Given this into account, patients who are not in line with the therapeutic target for LDL-C cholesterol should be encountered extremely rarely! To make this happen, continuous education must be promoted to avoid therapeutic inertia, improve adherence, and increase the availability of non-statin drugs (IA).
Pitavastatin – basic information and lipid-lowering effect
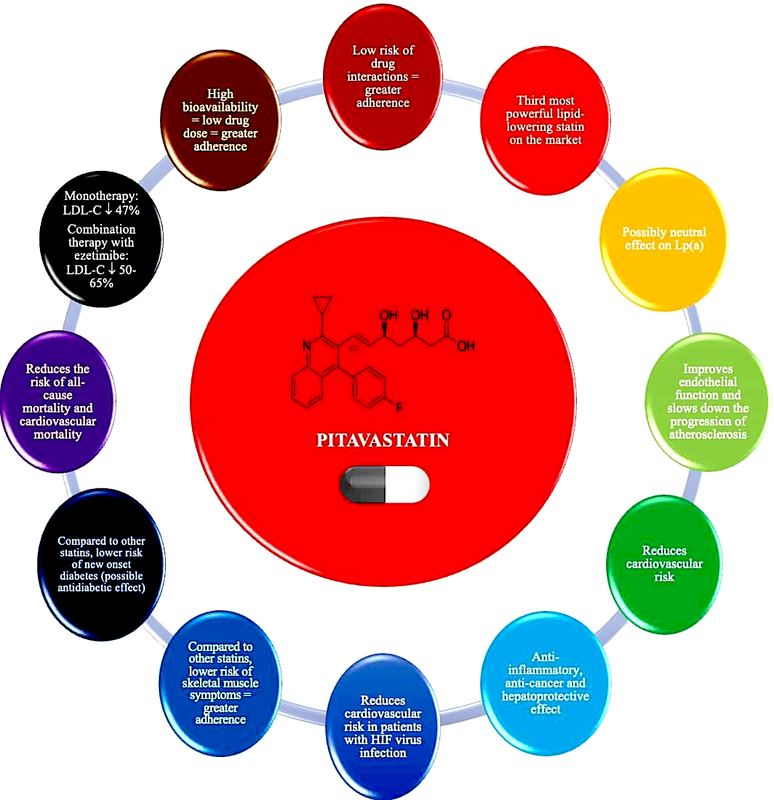
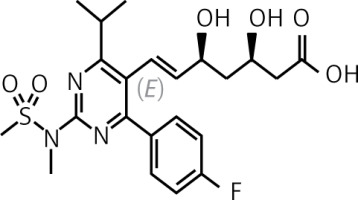
Pitavastatin is a lipophilic statin which has a very high bioavailability of 50% (43–51%) and is practically not metabolised in the body by the cytochrome P450 3A4 system (to a minimal extent – close to 3% – metabolised by CYP2C9 and CYP2C8), which significantly reduces the risk of interaction with other drugs (Table II) [47, 50, 51]. Pitavastatin does not exist as a prodrug, is 99% protein-bound in blood and its metabolites are inactive [51]. In an analysis by Gosho et al. involving three RCTs and real-world data, it was found that concomitant administration of pitavastatin with other medicines was not associated with a clinically significant increase in the incidence of adverse drug reactions, even when administered with agents that interact with CYP2C9, which is responsible for minimal metabolism of pitavastatin. In addition, a significant interaction of pitavastatin with biguanides was found, but this was associated with a reduced risk of muscle-related side effects [52]. The high bioavailability of pitavastatin means that it is administered at much lower doses (1, 2 or 4 mg) compared with rosuvastatin (5, 10, 20, 40 mg) or atorvastatin (10, 20, 40 80 mg), which can be used as an argument to convince patients who are concerned about high doses of these medicines (and reduce the risk of nocebo/drucebo effect [53]). The relatively long half-life of pitavastatin means that it is irrelevant at what time the medicine is taken, whether in the morning or in the evening [50].
Table II
Pitavastatin is the third most potent statin available on the Polish market in terms of lipid-lowering effect (Figure 5) [54–58]. A network meta-analysis including 50 RCTs (patients with dyslipidaemia, diabetes mellitus, ASCVD) by Zhang et al. compared seven statins in terms of their effects on individual lipid metabolism parameters. The results showed that rosuvastatin had the strongest effect on reducing serum LDL-C levels, followed by atorvastatin and pitavastatin [56]. The use of pitavastatin reduces serum total cholesterol by 29–33%, LDL-C by 42–50%, non-HDL-C by 41%, and triglycerides by 30–32%, and the effect is dose-dependent [57]. The LIVALO study showed that switching medication from pravastatin, simvastatin, fluvastatin or atorvastatin to pitavastatin was associated with increases in serum HDL-C of 21%, 11.8%, 20.1% and 15.8%, respectively [58]. Lipid-lowering treatment with pitavastatin is classified as moderately intensive or intensive [1, 27]. The use of pitavastatin 4 mg is equivalent to atorvastatin 20–40 mg or rosuvastatin 10–20 mg in terms of lipid-lowering effect, which allows for the personalisation of the achievement of therapeutic goals [1, 59].
The SCEAD study by Tarim et al., involving 180 patients with dyslipidaemia and type 2 diabetes, assessed the effects of pitavastatin, rosuvastatin and atorvastatin on the metabolic profile. Patients were randomised to pitavastatin 2 mg (n = 42), rosuvastatin 10 mg (n = 45) or atorvastatin 20 mg (n = 44) daily for 6 months. It was shown that the lipid-lowering potency of pitavastatin (reduction in serum concentrations of total cholesterol, LDL-C, and triglycerides) was not significantly different from rosuvastatin or atorvastatin. The increase in serum HDL-C was only significant in patients taking pitavastatin [60]. A randomised study by Hong et al. involving 1,101 post-ACS patients evaluated the effect of pitavastatin 2 mg or 4 mg/day for 12 months. It showed that pitavastatin 2 or 4 mg significantly reduced serum total cholesterol (43–55%) and LDL-C (34–42%) [61]. A RCT involving 106 children and adolescents with hypercholesterolaemia showed that the use of pitavastatin (1, 2 or 4 mg/day) for 52 weeks versus placebo was associated with a reduction in serum LDL-C of nearly 40% and was well tolerated [62]. The association of pitavastatin with ezetimibe is also beneficial. In a randomised trial by Jeong et al. involving 283 patients with lipid disorders, pitavastatin 2 mg + ezetimibe 10 mg/day or pitavastatin 2 mg/day or pitavastatin 4 mg/day + ezetimibe 10 mg/day were used for 8 weeks. The use of the combination of pitavastatin and ezetimibe was shown to reduce serum LDL-C by 52.8% (±11.2%), while pitavastatin alone reduced serum LDL-C by 37.1% (±14.1%) [63]. Another study involving 880 post-ACS patients using pitavastatin versus pitavastatin + ezetimibe for 3.4 years also showed a significantly stronger lipid-lowering effect of combination therapy for both LDL-C (66.4 ±21.7 vs. 85.1 ±23.1 mg/dl), total cholesterol (141 ±26 vs. 162 ±30 mg/dl) and triglycerides (134 ±76.7 vs. 155 ±98.1 mg/dl) [64]. The highly beneficial lipid-lowering effect of combining pitavastatin with ezetimibe was also confirmed in a meta-analysis of 9 RCTs in a group of 2,586 patients with CAD. It found that pitavastatin in combination with ezetimibe resulted in significantly greater reductions in serum LDL-C (SMD = –0.86; 95% CI: –1.15 to –0.58), total cholesterol –0.84; –1.10 to –0.59) and triglycerides (−0.59; –0.89 to –0.28) compared with pitavastatin monotherapy [65]. In a randomised clinical trial by Ihm et al., the effect of combination therapy with pitavastatin (2 mg/day) + fenofibrate (160 mg/day) vs. pitavastatin monotherapy (2 mg/day) was assessed in 347 patients with mixed dyslipidaemia. After 8 weeks of treatment, it was found that combination therapy was associated with greater reductions in serum non-HDL-C and LDL-C and increases in serum HDL-C (by –7.4%, –7.7% and +20.7%, respectively). Monotherapy and combination therapy were safe and well tolerated by patients [66]. It is worth noting that pitavastatin also improves the LDL-C subfraction profile, as it reduces the percentage of small LDL and oxidised LDL (oxLDL) [67].
Expert recommendation
Pitavastatin, due to its metabolism, has a very low risk of drug-drug interactions. It should be considered for use in patients requiring therapy with multiple medicines, especially those metabolised by the CYP3A4 enzyme in cytochrome P450. Due to its highest bioavailability among statins, it is used in lower doses, which may improve adherence, and has a moderately potent lipid-lowering effect (it is the third most potent statin enabling LDL cholesterol reduction of up to 47%) (IA).
Pitavastatin – effect on Lp(a)
Elevated serum Lp(a) concentration is an ASCVD risk factor independent of serum LDL-C concentration [68]. The EAS consensus indicates that the cut-off value for serum Lp(a) concentration should be 50 mg/dl (−125 nmol/l) [46]. However, there are data indicating that already at serum Lp(a) concentrations above 30 mg/dl (−75 nmol/l), CV risk increases [1, 68]. There are also indications of a preference for tests that determine the number of Lp(a) molecules in nmol/l rather than the mass in mg/dl [1].
The use of statins is associated with an increase in serum Lp(a) concentrations, although this is not clinically relevant [69, 70]. On average, the increase in serum Lp(a) concentration is 6–10% [1]. The increase in serum Lp(a) levels in patients treated with statins is part of the residual CV risk that remains, despite good control of serum LDL-C levels [69, 71].
In the context of its effect on Lp(a) concentration, pitavastatin also appears to be different from other statins (no class effect), as it does not affect, or may even slightly reduce its serum concentration. The VISION randomised clinical trial evaluated the effect of pitavastatin 2 mg/day vs. atorvastatin 10 mg/day for 12 weeks on serum Lp(a) concentrations in 42 patients with hypercholesterolaemia. It was concluded that pitavastatin showed a tendency to slightly reduce serum Lp(a) concentrations, although this effect did not reach statistical significance. No such effect was shown for atorvastatin [72]. These findings were confirmed in a meta-analysis of 20 RCTs conducted by Wang et al. involving 23,605 participants. In this meta-analysis, it was found that, of all statins, pitavastatin had the most beneficial effect on serum Lp(a) concentrations, which was manifested by a tendency to reduce these concentrations [73].
Expert recommendation
Pitavastatin, unlike other statins, appears to have a neutral effect on serum Lp(a) concentrations, and may even reduce these concentrations to a small extent. Pitavastatin may be considered for use in patients with elevated Lp(a) concentrations (IIb). Further studies are needed to confirm this relationship, as well as to assess the effect of pitavastatin on the size of apolipoprotein(a) isoforms.
Pitavastatin – effects on cardiovascular risk and prognosis
In addition to its proven lipid-lowering effect, pitavastatin improves vascular endothelial function. A meta-analysis of six studies by Katsiki et al. showed that pitavastatin use was associated with improved vascular endothelial function, assessed as flow-mediated dilation (FMD). Pitavastatin significantly increased FMD (WMD = 2.45%; 95% CI: 1.31–3.60) [74]. The Pitavastatin Evaluation of Atherosclerosis Regression by Intensive Cholesterol-lowering Therapy (PEACE) randomised clinical trial involving 303 patients with carotid intima-media thickness (CIMT) (> 1.1 mm) and serum LDL-C levels > 100 mg/dl (2.5 mmol/l) assessed the effect of moderate or intensive pitavastatin treatment on atherosclerosis regression. After 12 months of follow-up, it was shown that more intensive treatment with pitavastatin was associated with a reduction in CIMT of 0.024 mm (95% CI: –0.046 to –0.0014) [75]. The JAPAN-ACS randomised clinical trial (307 patients with ACS) assessed the effect of pitavastatin and atorvastatin on coronary artery plaque volume. It was found that both drugs significantly reduced serum LDL-C and non-HDL-C concentrations and reduced atherosclerotic plaque volume (respectively: LDL-C –36.2 ±19.5% and –35.8± 22.9% and non-HDL-C –30.5 ±18.9% and –30.1 ±20.8%; coronary atherosclerotic plaque volume –16.9± 13.9% and –18.1± 14.2%) in coronary arteries during the 10-month intervention [76].
The beneficial effect of pitavastatin translates into a reduction in CV risk. The REAL-CAD study, which included patients with stable CAD, assessed the efficacy and safety of pitavastatin. Patients were randomised to pitavastatin 4 mg/day (n = 6,526) or 1 mg/day (n = 6,528), with a follow-up time of 3.9 years. More intensive treatment with pitavastatin was shown to be associated with a reduced risk of death from any cause by 19% (HR = 0.81; 95% CI: 0.68–0.98), ACS by 43% (HR = 0.57; 95% CI: 0.38–0.83) and the need for coronary revascularisation by 14% (HR = 0.86; 95% CI: 0.76–0.96) [77]. Another study involving 664 patients with hypercholesterolaemia and a high risk of ASCVD assessed the effect of pitavastatin 2 mg/day vs. atorvastatin 10 mg/day on the risk of a primary endpoint including cardiovascular death, sudden death of unknown origin, non-fatal ACS, non-fatal stroke, transient ischaemic attack or heart failure requiring hospitalisation, and a secondary endpoint representing the combination of the primary endpoint and clinically indicated coronary revascularisation for stable angina [78]. After 240 days of intervention, it was shown that the lipid-lowering effect was not significantly different between pitavastatin and atorvastatin, whereas pitavastatin reduced the risk of both the primary and secondary endpoints to a greater extent (HR = 0.37; 95% CI: 0.17–0.79 and HR = 0.35; 95% CI: 0.19–0.65, respectively) [78]. A study by Yu et al. that included 427,407 patients with type 2 diabetes using statins, and 422,380 patients with type 2 diabetes not using statins, assessed the effect of these drugs on the risk of death from any cause. A follow-up of up to 9 years showed that patients with type 2 diabetes who used statins had a significantly lower risk of death from any cause (HR = 0.32; 95% CI: 0.31–0.33) [79]. Hierarchical comparative analysis showed that the protective effect against mortality was greatest with pitavastatin, followed by rosuvastatin, pravastatin, simvastatin, atorvastatin, fluvastatin and finally lovastatin [79]. These results may be related to the metabolic properties of pitavastatin, which, in addition to reducing LDL-C, significantly improves atherogenic dyslipidaemia (with the greatest effect on HDL-C and TG), and may also slightly reduce fasting glucose, glycated haemoglobin and improve the HOMA-IR index [1]. The study by the same authors involving the same patient group and intervention found that the use of pitavastatin was associated with the greatest reduction in the risk of CV death (HR = 0.11; 95% CI: 0.06–0.22) [80]. Another RCT involving 848 haemodialysis patients showed that during a follow-up of 36.5 months, those who used pitavastatin had a lower risk of the primary endpoint (including death from any cause and ACS; p = 0.007) as well as the composite endpoint (including rates of coronary intervention, stroke, fractures and hospitalisation for heart failure and unstable angina; p = 0.022) [81].
As for other statins, the combination of pitavastatin with ezetimibe also has a favourable effect on prognosis. The Otsuki et al. study, which enrolled 880 post-ACS STEMI patients and used pitavastatin vs. pitavastatin + ezetimibe for 3.4 years, showed that combination therapy was associated with a reduction in the risk of death from any cause by 55% (HR = 0.45; 95% CI: 0.23–0.84) and non-fatal stroke by 23% (HR = 0.77; 95% CI: 0.62–0.97) [64].
Pitavastatin – impact on new cases of diabetes mellitus
The use of statins is associated with a small increase in the risk of new cases of diabetes (new onset diabetes, NOD), especially when used at high doses [47]. Nevertheless, it must be noted that this risk is 5 times lower than the benefit associated with the reduction in CVD events with statin treatment [82].
The aforementioned study by Hong et al. involving 1,101 post-ACS patients showed that fasting plasma glucose levels were significantly reduced in both groups taking pitavastatin (4 mg/day: –20.16 ±54.49 mg/dl and 2 mg: –24.45 ±63.88 mg/dl) [61]. In a study by Liu et al., in 8,337 patients on moderately intensive statin therapy (pitavastatin 2 mg/day; atorvastatin 10 mg/day; rosuvastatin 10 mg/day) who were followed for 4 years, it was shown that pitavastatin had the least diabetogenic effect [83]. The real world evidence (RWE) study analysis by Seo et al. included 10,238 patients using pitavastatin and 18,605 using atorvastatin or rosuvastatin. They found that, compared with atorvastatin and rosuvastatin, pitavastatin use was associated with a lower risk of NOD (HR = 0.69; 95% CI: 0.54–0.88 and HR = 0.74; 95% CI: 0.55–0.99, respectively) [84]. A prospective study by Jeong et al., with 667 post-ACS patients at high risk of developing type 2 diabetes, evaluated the effect of pitavastatin 2 and 4 mg/day on the risk of diabetes. A follow-up of 3 years showed that the NOD risk did not differ between patients using pitavastatin 2 mg/day vs. 4 mg/day (p = 0.36) [85]. Similar results were obtained in a study by Lee et al. involving 313 patients with hypercholesterolaemia and abnormal fasting plasma glucose levels who were followed for 1 year, in which the results showed that more intensive pitavastatin therapy (4 mg/day) vs. less intensive therapy (2 mg/day) was not associated with an increased risk of new cases of diabetes (p = 0.43) [86]. A meta-analysis of 15 randomised trials by Vallejo-Vaz et al. showed that the use of pitavastatin did not affect fasting plasma glucose levels (MD = –0.01 mg/dl; 95% CI: –0.7–0.74), glycated haemoglobin percentage (MD = –0.03%; 95% CI: –0.11–0.05) as well as the NOD risk (RR = 0.70; 95% CI: 0.30–1.60) [87]. In the aforementioned SCEAD study involving 180 patients with dyslipidaemia and type 2 diabetes, which assessed the effects of pitavastatin, rosuvastatin and atorvastatin on the metabolic profile, it was found that, of the statins tested, only pitavastatin significantly reduced glycated haemoglobin percentage (−0.75%) and fasting plasma glucose (−19.0 mg/dl; 95% CI: –40.0 to –1.5) [60]. The inhibition of hepatocyte cellular proliferation and the inhibition of phosphatidylinositol kinase (PI3K), which leads to a stimulation of glycogenogenesis in the liver, may be responsible for the beneficial effect of pitavastatin on glycaemic control observed in studies [88].
Expert recommendation
Pitavastatin does not affect the risk of new cases of diabetes and may even improve plasma glucose control, glycated haemoglobin and HOMA-IR in patients with metabolic disorders and diabetes. Pitavastatin should be considered in patients at risk of diabetes and with diabetes to optimise treatment of both lipid disorders and antidiabetic therapy (IIa).
Pitavastatin and statin-associated muscle symptoms (SAMS)
The most reported adverse event of statins is myalgia. They represent the main reason for non-adherence to statin therapy [89–91]. However, it should be emphasised that the actual incidence of post-statin myalgia is low [47].
The study by Moroi et al., involving 664 patients with hypercholesterolaemia and high ASCVD risk, showed that pitavastatin use was associated with a significantly lower incidence of myalgia compared with atorvastatin (1.3% vs. 3.9%, p = 0.036) [78]. Similar results were obtained in a study involving 1,101 post-ACS patients, which assessed the effect of pitavastatin 2 mg or 4 mg/day for 12 months. The incidence of myalgia was even lower in patients using pitavastatin 4 mg/day (1.7% vs. 2.6%) [61]. In the REAL-CAD randomised clinical trial, the incidence of myalgia was 0.7% with pitavastatin 1 mg/day and 1.9% with 4 mg/day, which was comparable to the placebo group [77]. The observed effect may be due, among other things, to a more favourable effect of pitavastatin on coenzyme Q10 levels in the body. A RCT by Kawashiri et al. involving 19 patients with heterozygous familial hypercholesterolaemia (heFH) compared the effect of pitavastatin (4 mg/day) vs. atorvastatin (20 mg/day) for 16 weeks on plasma coenzyme Q10 levels. It was shown that, compared with atorvastatin, pitavastatin did not significantly affect plasma coenzyme Q10 concentrations (−7.7% vs. –26.1%, p < 0.03) [92]. The fact that pitavastatin is used at low doses (1–4 mg) compared to other statins, which many patients interpret as a lower risk of adverse effects, may be important in improving adherence and reducing the nocebo/drucebo effect.
Pitavastatin – effects on other parameters
Pitavastatin, like other statins, is characterised by its anti-inflammatory effect. A previously cited study by Otsuki et al. involving 880 post-ACS patients showed that the use of pitavastatin as well as pitavastatin with ezetimibe significantly reduced plasma hsCRP levels (pitavastatin: 27.3 ±35.5 vs. 2.45 ±8.18; pitavastatin + ezetimibe: 26.6 ±33.7 vs. 1.52 ±3.28) after 3.4 years of intervention [64].
A study by Nagayama et al. that included 622 patients with hypercholesterolaemia and a high risk of ASCVD showed that the use of pitavastatin (2 mg/day) compared with atorvastatin (10 mg/day) for 240 weeks was associated with a lower risk of new cancers (0.32% vs. 1.94%, p = 0.051; although neither statin significantly increased this risk, showing mostly protective effects) [93]. A meta-analysis of 32 studies by Wang et al. involving nearly 5 million people showed that, of the so-called potent statins, pitavastatin reduced the risk of hepatocellular carcinoma to the greatest extent (OR = 0.36; 95% CI: 0.17–0.75), demonstrating the strong hepatoprotective properties of this statin [94]. In conclusion, pitavastatin has anti-inflammatory effects and may also exhibit anticancer and hepatoprotective effects.
Pitavastatin – HIV-infected patients
Patients with acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV) have a higher risk of lipid disorders and ASCVD compared to members of the general population [95]. Furthermore, this group has a higher risk of developing CVD and treatment may be more difficult, as many of the medicines used in therapy use the cytochrome P450-related pathway.
The randomised clinical trial of HIV-infected patieNts and TREatment with PItavastatin vs pravastatin for Dyslipidemia (INTEREPID) by Aberg et al. involving 252 HIV-1-infected and dyslipidaemic patients showed that pitavastatin (4 mg/day) compared to pravastatin (40 mg/day) administered over 52 weeks improved the lipid profile of these patients to a significantly greater extent (total cholesterol: –19.1% vs. –13.7%; LDL-C: –29.7% vs. –20.5%; non-HDL-C: –26.1% vs. 19.0%; apoB: –25.4% vs. –19.6%) with a similar safety and tolerability profile [96]. The results of this study formed the basis for the Randomised Trial to Prevent Vascular Events in HIV (REPRIEVE), a randomised clinical trial involving 7,769 patients with HIV infection. In this study, pitavastatin 4 mg/day or placebo was used, and the follow-up period was 5.1 years. It was shown that the use of pitavastatin in HIV-infected patients was associated with a reduction in the risk of MACE by up to 35% (HR = 0.65; 95% CI: 0.48–0.90). Pitavastatin was also well tolerated by these patients (risk of adverse events: IRR = 1.06; 95% CI: 0.98–1.15) [97].
Pitavastatin – summary of recommendations
The properties of pitavastatin outlined above make this drug a valuable tool in optimising and, above all, personalising lipid disorder therapy in many patient groups (Figures 5 and 6). Pitavastatin may be an important medicine for optimising the treatment of lipid disorders in patients with an increased risk of type 2 diabetes, as well as already existing type 2 diabetes, as indicated by the International Lipid Expert Panel (ILEP) in its 2022 position statement [98]. Based on pitavastatin, lipid-lowering treatment can be gradually intensified in the above patients: pitavastatin (LDL-C↓ by about 50%); pitavastatin + ezetimibe (LDL-C↓ by about 50–65%), pitavastatin + ezetimibe + bempedoic acid (LDL-C↓ by about 65–80%), pitavastatin + ezetimibe + PCSK9 modulator (LDL-C↓ by > 80%) or pitavastatin + ezetimibe + bempedoic acid + PCSK9 modulator (LDL-C↓ by > 80%), and additionally increase type 2 diabetes treatment control [98]. This agent should also be considered in statin-intolerant patients presenting with muscle pain, as the risk of SAMS after pitavastatin is comparable to placebo. The 2021 guidelines of the Polish Lipid Association (PoLA), and five other scientific societies, indicate that pitavastatin can be used in patients with: 1) low CV risk (expected LDL-C fall: < 30%) at 1 mg/day; 2) moderate CV risk (expected LDL-C fall: 30–50%) at 2–4 mg/day; and 3) high CV risk (expected LDL-C fall: 50–60%) at 4 mg/day in combination with ezetimibe 10 mg/day [1]. Despite the small amount of available data (still often conflicting), the use of pitavastatin may be considered in patients with lipid disorders accompanied by elevated serum Lp(a) levels. This may allow optimisation of residual risk in such patients. However, these properties of pitavastatin require confirmation in ongoing RWE studies [72, 73]. Finally, studies conducted with HIV-infected patients allow indicating pitavastatin as the statin of first choice in this group of patients [96, 97].
In conclusion, pitavastatin, effectively complements our treatment options for patients with lipid disorders and in patients with comorbidities and side effects, allowing us to personalise the treatment with/without non-statin drugs.