Introduction
Immunotherapy based on monoclonal antibodies against immune-checkpoint proteins has achieved success in many types of cancer, attracting the attention of the medical community. In particular, monoclonal antibodies against programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen 4 (CTLA-4) have been recently approved for patients with cancer [1].
Randomized controlled trials (RCTs) evaluating the efficacy of combined immunotherapy are also increasingly reported in many types of cancer. The combination of nivolumab and ipilimumab showed longer survival time and progression-free survival than either nivolumab or ipilimumab in advanced melanoma, non-small cell lung cancer, renal cell carcinoma, and microsatellite instability (MSI)-high cancers. Similarly, the combination of durvalumab and tremelimumab achieved better results than each individually in malignant pleural mesothelioma, non-small cell lung cancer, metastatic pancreatic ductal adenocarcinoma, and recurrent or metastatic head/neck squamous cell carcinoma, attributed to the dual inhibitory effects of PD-1/PD-L1 and CTLA-4 pathways, which may enhance the antitumour efficacy [1]. In many cancer patients, these monoclonal antibodies have shown significant antitumour activities, including tumour reduction and increasing progression-free survival or overall survival. Although immune-mediated side effects have been reported in up to 90%, these are generally manageable, with a low frequency of fatal or fulminant adverse events [1–4]. Some clinical trials show that combining 2 immunotherapy drugs may be associated with a higher incidence of death than a single immunotherapy drug [5]. The magnitude of the effects of immunotherapy drugs in cancer patients on adverse cardiac events are unknown [3–5].
We systematically evaluated the impact of combined immunotherapy (PD-1 inhibitors plus CTLA-4 inhibitors or PD-L1 inhibitors plus CTLA-4 inhibitors) versus single immunotherapy on the risk of severe cardiac events.
Material and methods
This systematic review was reported following the recommendations of the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [6]. The protocol for this systematic review and meta-analysis was registered in PROSPERO (CRD42021283946) (Supplementary Table SI).
Study searches
We searched the following electronic databases: PubMed, Embase, Scopus, and Web of Science, from inception to 26 August 2021. The complete search strategy can be found in Supplementary Table SII. There was no restriction on publication year or language. The reference lists of included studies and relevant reviews were also screened to identify eligible studies. The obtained articles were downloaded, and duplicates were removed.
Inclusion and exclusion criteria
The selection of studies was independently performed by 2 authors (GVR, JAC); discrepancies were resolved by discussion. We included published phase III RCTs that compared combined immunotherapy (PD-1 inhibitors plus CTLA-4 inhibitors, or PD-L1 inhibitors plus CTLA-4 inhibitors) with single immunotherapy (PD-1 inhibitors, PD-L1 inhibitors, or CTLA-4 inhibitors). Pre-specified PD-1 inhibitors included pembrolizumab, nivolumab, and cemiplimab; PD-L1 inhibitors included atezolizumab, avelumab, and durvalumab; and CTLA-4 inhibitors included ipilimumab and tremelimumab. The following types of cancers were of interest: melanoma, oesophagogastric carcinoma, renal cell carcinoma, and non-small cell lung cancer. These types of cancer were selected because immunotherapy agents are commonly prescribed among them [1].
Data extraction
Data were extracted using a pre-determined spreadsheet in Microsoft Excel. Two reviewers (GVR, JAC) independently extracted data; any disagreement in extractions was resolved by a third review author (CDA). The following data were extracted: first author’s name, year of publication, type of cancer population treated, mean age, percentage of men, follow-up duration, and primary and secondary outcomes per treatment arm.
Outcomes
Our pre-specified primary outcomes were acute coronary syndrome, myocardial infarction, heart failure, and atrial fibrillation. Secondary outcomes were atrial flutter, cardiac tamponade, cardiac arrest, and bradycardia. We used the definition of cardiac adverse events as reported by the authors.
Quality assessment and statistical analyses
The same 2 reviewers (GVR, JAC) assessed the risk of bias (RoB) using the Cochrane RoB 2.0 tool [7]. Each RCT was rated as having a high risk of bias, some concerns, or a low risk of bias. Any disagreement was resolved by consensus.
All meta-analyses were conducted using a random-effects model with the inverse variance method. The Paule-Mandel method was used to calculate between-study variance (τ2) [8]. Effects were expressed as relative risks (RR) and their corresponding 95% confidence intervals (CI). A treatment arm continuity correction for zero events was performed [9]. In addition, the proportions of all outcomes in each group (combined and single therapy) with their 95% CI were pooled using the Freeman-Tukey double arcsine transformation. Statistical heterogeneity was evaluated using the I2 statistic and was defined as follows: I2 > 30% indicates mild, 30–60% moderate, and > 60% high heterogeneity [10]. We evaluated small study effects using funnel plots and Egger’s test when 10 or more studies were available per outcome [11]. We used the meta package from R 4.1.2 (www.r-project.org) for all meta-analyses. A 2-tailed p < 0.05 was considered statistically significant.
GRADE certainty of evidence
The certainty of evidence (CoE) was evaluated using the GRADE approach [12]. The CoE per outcome includes the analysis of 5 domains: RoB, inconsistency, imprecision, indirectness, and publication bias, and the complete CoE table was presented in the summary of findings (SoF) table using GRADEpro software (McMaster University and Evidence Prime, 2021; www.gradepro.org/).
Results
Study selection
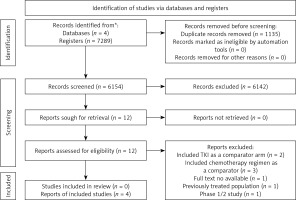
A total of 7289 articles were evaluated. After excluding 7277 articles, we retrieved the full texts of 12 studies for detailed evaluation. Eight studies were excluded: 2 including tyrosine kinase inhibitors (TKIs) as comparators, 3 including chemotherapy as comparators, one where full-text was unavailable, one including previously treated patients, and one was a phase 1/2 study. Finally, 4 RCTs (n = 1581) were included for analysis [13–16] (Figure 1).
Characteristics of included RCTs
The 4 included RCTs were published between 2015 and 2020. Drugs being compared included durvalumab versus durvalumab plus tremelimumab, nivolumab versus nivolumab plus ipilimumab, durvalumab versus durvalumab plus tremelimumab, and ipilimumab versus ipilimumab plus nivolumab.
The mean age was between 59 and 67 years. The male gender was found between 63.9% and 84.6% across trials. Follow-up times among RCTs ranged from 18 to 39 months.
The most commonly reported severe cardiac outcomes were atrial fibrillation (13 adverse events, 3 RCTs), heart failure (6 adverse events, 3 RCTs), and acute coronary syndromes (5 adverse events, 4 RCTs) across trials. The types of cancer evaluated were melanoma (2 RCTs), head and neck squamous cell carcinoma (one RCT), and non-small cell lung cancer (one RCT).
Acute coronary syndromes were reported in 3.66 per 1000 people with combined immunotherapy and in 2.62 per 1000 people with single immunotherapy. Cases of myocardial infarction were only reported in 3.66 per 1000 people with combined immunotherapy. Cases of heart failure were reported in 6.11 per 1000 people with combined immunotherapy and in 1.31 per 1000 people with single immunotherapy. Cases of atrial fibrillation were reported in 12.22 per 1000 people with combined immunotherapy and in 3.99 per 1000 people with single immunotherapy. Atrial flutter cases were reported in 3.66 per 1000 people with combined immunotherapy and in 1.31 per 1000 people with single immunotherapy. No cases of bradycardia were reported with combined immunotherapy, and 1.31 per 1000 people were reported with single immunotherapy. Cases of cardiac arrest only were reported in 2.44 per 1000 people with combined immunotherapy. Finally, cases of cardiac tamponade were reported in 1.22 per 1000 people with combined immunotherapy, and in 1.31 per 1000 people with single immunotherapy (Table I).
Table I
Characteristics of included randomized controlled trials
| Trial information | Adverse events | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study reference | Age | Male gender | Sample | Follow-up Time [months] | Type of cancer | Acute coronary syndromes | Myocardial infarction | Heart failure | Atrial fibrillation | Atrial flutter | Bradycardia | Cardiac arrest | Cardiac tamponade |
| Ferris, 2020 [13]: | Median age | No (%) | |||||||||||
| Intervention: Durvalumab plus Tremelimumab | 61 | 209 (84.6%) | 247 | 34 | Head and neck squamous cell carcinoma | 2 (0.81%) | NR | 1 (0.41%) | NR | 1 (0.41%) | NR | 1 (0.41%) | NR |
| Control: Durvalumab | 59 | 202 (84.2%) | 240 | 0 (0%) | NR | 0 (0%) | NR | 0 (0%) | NR | 0 (0%) | NR | ||
| Larkin, 2015 [14]: | Mean age | No (%) | |||||||||||
| Intervention: Nivolumab plus Ipilimumab | 59 | 206 (65.6%) | 313 | 21 | Melanoma | 0 (0%) | 1 (0.32%) | 1 (0.32%) | 4 (1.28%) | 1 (0.32%) | 0 (0%) | 0 (0%) | 1 (0.32%) |
| Control: Nivolumab | 59 | 202 (63.9%) | 313 | 1 (0.32%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.32%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Rizvi, 2020 [15]: | Median age | No (%) | |||||||||||
| Intervention: Durvalumab mas tremelimumab | 64 | 113 (69.3%) | 163 | 39 | Non small cell lung cancer | 1 (0.27%) | 2 (0.54%) | 1 (0.27%) | 5 (1.35%) | 1 (0.27%) | 0 (0%) | 1 (0.27%) | 0 (0%) |
| Control: Durvalumab | 65 | 118 (72.4%) | 163 | 0 (0%) | 0 (0%) | 1 (0.27%) | 2 (0.54%) | 0 (0%) | 1 (0.27%) | 0 (0%) | 1 (0.27%) | ||
| Postow, 2015 [16]: | Median age | No (%) | |||||||||||
| Intervention; Nivolumab plus Ipilimumab | 64 | 63 (66%) | 95 | 18 | Melanoma | NR | NR | NR | 1 (1.6%) | NR | NR | NR | NR |
| Control: Ipilimumab | 67 | 32 (68%) | 47 | NR | NR | NR | 1 (2.17%) | NR | NR | NR | NR | ||
Risk of bias assessment
All RCTs were assessed as having some concerns of bias (Supplementary Figure S1). All of them had some concerns of bias in the domain of selection of the reported result. One study had additional concerns in the domain of randomization, and one in the domain of measurement of the outcome.
Effects of combined immunotherapy on primary outcomes
Acute coronary syndromes
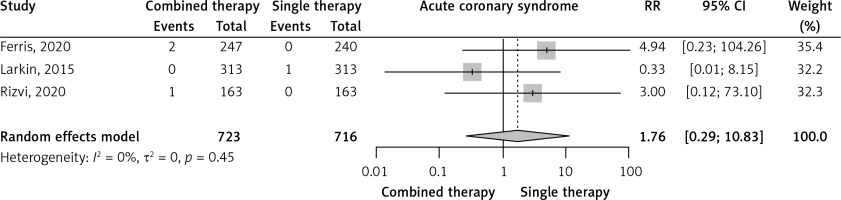
Three RCTs reported the incidence of acute coronary syndromes. Combined immunotherapy non-significantly increased acute coronary syndromes compared to single immunotherapy (RR = 1.76, 95% CI: 0.29–10.83, I2 = 0%, Figure 2). The prevalence of acute coronary syndromes was very low in the combined immunotherapy arms (0.3%, 95% CI: 0–1.2%, I2 = 42%) and in the single immunotherapy arms (0.1%, 95% CI: 0–0.5%, I2 = 0%) (Supplementary Figures S8 and S16).
Myocardial infarction
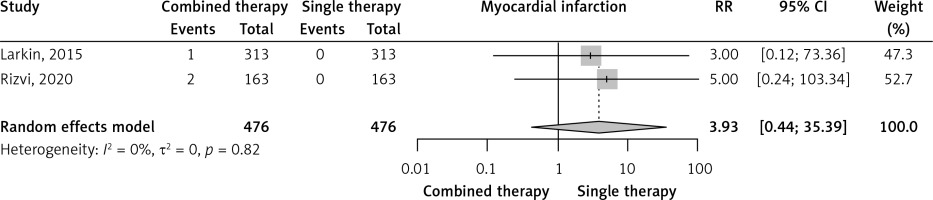
Two RCTs reported the incidence of myocardial infarction. Combined immunotherapy non-significantly increased myocardial infarction compared to single immunotherapy (RR = 3.93, 95% CI: 0.44–35.39, I2 = 0%, Figure 3). The prevalence of myocardial infarction was very low in combined immunotherapy arms (0.6%, 95% CI: 0–1.8%, I2 = 23%) and in single immunotherapy arms (0%, 95% CI: 0–0.4%, I2 = 0%) (Supplementary Figures S9 and S17).
Heart failure
Three RCTs reported the incidence of heart failure. Combined immunotherapy non-significantly increased heart failure compared to single immunotherapy (RR = 2.99, 95% CI: 0.61–14.79, I2 = 0%, Supplementary Figure S2). The prevalence of heart failure was very low in the combined immunotherapy arms (0.6%, 95% CI: 0–1.6%, I2 = 29%) and in the single immunotherapy arms (0.04%, 95% CI: 0–0.49%, I2 = 5%) (Supplementary Figures S10 and S18).
Atrial fibrillation
Three RCTs reported the incidence of atrial fibrillation. Combined immunotherapy non-significantly increased atrial fibrillation compared to single immunotherapy (RR = 2.26, 95% CI: 0.62–8.16, I2 = 2%, Supplementary Figure S3). Prevalence of atrial fibrillation was very low in the combined immunotherapy arms (1.6%, 95% CI: 0.7–2.9%, I2 = 0%) and in the single immunotherapy arms (0.4%, 95% CI: 0–2.6%, I2 = 67%) (Supplementary Figures S11 and S19).
Effects of combined immunotherapy on secondary outcomes
Atrial flutter
Three RCTs reported the incidence of atrial flutter. Combined immunotherapy non-significantly increased atrial flutter compared to single immunotherapy (RR = 1.93, 95% CI: 0.33–11.11, I2 = 0%, Supplementary Figure S4). The prevalence of atrial flutter was very low in the combined immunotherapy arms (0.4%, 95% CI: 0–1.1%, I2 = 0%) and in the single immunotherapy arms (0.1%, 95% CI: 0–0.5%, I2 = 0%) (Supplementary Figures S12 and S20).
Cardiac tamponade
Two RCTs reported the incidence of cardiac tamponade. Combined immunotherapy non-significantly increased cardiac tamponade compared to single immunotherapy (RR = 1.0, 95% CI: 0.10–9.56, I2 = 0%, Supplementary Figure S5). The prevalence of cardiac tamponade was very low in the combined immunotherapy arms (0.1%, 95% CI: 0–0.8%, I2 = 0%) and in the single immunotherapy arms (0.1%, 95% CI: 0–1.2%, I2 = 47%) (Supplementary Figures S13 and S21).
Cardiac arrest
Three RCTs reported the incidence of cardiac arrest. Combined immunotherapy non-significantly increased cardiac arrest compared to single immunotherapy (RR = 2.27, 95% CI: 0.32–16.12, I2 = 0%, Supplementary Figure S6). The prevalence of cardiac arrest was very low in the combined immunotherapy arms (0.2%, 95% CI: 0–0.8%, I2 = 14%) and in the single immunotherapy arms (0%, 95% CI: 0–0.3%, I2 = 0%) (Supplementary Figures S14 and S22).
Bradycardia
Two RCTs reported the incidence of bradycardia. Combined immunotherapy non-significantly increased bradycardia compared to single immunotherapy (RR = 0.52, 95% CI: 0.04–6.14, I2 = 0%, Supplementary Figure S7). The prevalence of bradycardia was very low in the combined immunotherapy arms (0%, 95% CI: 0–0.4%, I2 = 0%) and in the single immunotherapy arms (0.1%, 95% CI: 0–1.2%, I2 = 47%) (Supplementary Figures S15 and S23).
GRADE summary of findings
All primary and secondary outcomes were judged as having very low certainty of evidence (Table II).
Table II
Summary of findings (SOF) table for primary and secondary outcomes
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with single immunotherapy | Risk with combined immunotherapy | ||||
| Acute coronary syndromes follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 2) | RR = 1.76 (0.29–10.83) | 1439 (3 RCTs) | ⨁○○○ Very lowa,b |
| Myocardial infarction follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 0) | RR = 3.93 (0.44–35.39) | 952 (2 RCTs) | ⨁○○○ Very lowc,d |
| Heart failure (HF) follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 2) | RR = 2.99 (0.61–14.79) | 1439 (3 RCTs) | ⨁○○○ Very lowa,b |
| Atrial fibrillation follow-up: range 18 months to 39 months | 1 per 100 | 1 per 100 (0 to 5) | RR = 2.26 (0.62–8.16) | 1094 (3 RCTs) | ⨁○○○ Very lowb,e |
| Atrial flutter follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 2) | RR = 1.93 (0.33–11.11) | 1439 (3 RCTs) | ⨁○○○ Very lowa,b |
| Cardiac tamponade follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 2) | RR = 1.00 (0.10–9.56) | 952 (2 RCTs) | ⨁○○○ Very lowb,c |
| Cardiac arrest follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 0) | RR = 2.27 (0.32-16.12) | 1439 (3 RCTs) | ⨁○○○ Very lowa,d |
| Bradycardia follow-up: range 21 months to 39 months | 0 per 100 | 0 per 100 (0 to 1) | RR = 0.52 (0.04–6.14) | 952 (2 RCTs) | ⨁○○○ Very lowb,c |
a RoB2.0: Ferris et al. had some concerns of bias in the randomization process and the selection of the reported result. Rizvi et al. had some concerns of bias in the measurement of the outcome and the selection of the reported result. Larkin et al. had some concerns in the selection of the reported result.
Discussion
This meta-analysis, including 4 RCTs and 1581 patients, showed that the risk of cardiac adverse events was similar between patients treated with combined immunotherapy compared to single immunotherapy, including follow-up periods from 21 to 39 months. Moreover, the prevalence of adverse cardiac events in each group was very low across the studies.
Cardiotoxicity is defined as any heart compromise (functional or structural) related to disease treatment. Immunotherapy-associated cardiotoxicity is not a common complication, but it is usually serious and associated with high mortality rates [3, 4, 17]. Its incidence ranges from 0.09% to 3.17% of patients, but these rates are most likely underestimated [17].
The first specific case of immunotherapy-associated cardiotoxicity was published in 2014 [5]. Since then, there has been a gradual increase in the number of cases. This type of toxicity includes all parts of the heart, having inflammatory effects (myocarditis, pericarditis, left ventricular dysfunction without myocarditis) and non-inflammatory effects (asymptomatic non-inflammatory left ventricular dysfunction, Takotsubo-like syndrome, coronary vasospasm, arrhythmias, and myocardial infarction) [5].
The mechanism of immunotherapy-associated cardiotoxicity is not yet fully understood. It involves the infiltration predominantly of CD4+/CD8+ T lymphocytes and secondarily of macrophages (CD68+ cells) and the increase of inflammatory molecule levels such as tumour necrosis factor-α, granzyme β, and interferon-γ, all inducing cell death. Another mechanism may be a “shared antigen” between the tumour and cardiac cells, with muscle-specific antigens (desmin and troponin) detected in the tumour. Moreover, cardiomyocytes may also employ PD-1/PD-L1 and CTLA-4 pathways to prevent T cells from hyperactivation in physiological conditions. Then, these new molecules are used for cancer treatment, liberating the T-cell inhibition by tumour cells and the suppression in cardiomyocytes, leading to T-cell hyperactivation in the heart [5, 18].
The use of immunotherapy increased the number of cardiac adverse events, as was demonstrated by Nso et al. [4] with information from 26 RCTs (6 with combined immunotherapy, 10 with single immunotherapy, and 10 with a combination of immunotherapy and other antineoplastic drugs). The incidence of immune-related adverse events was as follows: myocarditis: 0.5% (95% CI: 0.1–0.9%), pericardial effusion: 0.5% (95% CI: 0.1–1%), heart failure: 0.3% (95% CI: 0.1–0.5%) atrial fibrillation: 4.6% (95% CI: 1–14.1%); myocardial infarction: 0.4% (95% CI: 0.0–0.7%), and cardiac arrest: 0.4% (95% CI: 0.1–0.8%).
In our meta-analysis of 4 RCTs, including patients with melanoma, oesophagogastric carcinoma, renal cell carcinoma, and non-small cell lung cancer, we found that the risk of severe cardiac adverse events with combination immunotherapy seems similar to single immunotherapy, but the evidence was very uncertain. Overall, these results add to the evidence of the cardiovascular safety of immunotherapy drugs. However, we need more clinical trials and prospective real-world registries to better establish the incidence and prognosis of these cardiac adverse events.
The use of immunotherapy may be associated with an increased number of severe cardiac events, but there are not many studies that quantify this association. Li et al. [3] in a previous meta-analysis of 18 RCTs with 11,394 patients with non-small cell lung cancer demonstrated that the use of anti-PD-1/PD-L1 as single immunotherapy increased the frequency of pericardial effusion (RR = 2.72, 95% CI: 1.45–5.12, p = 0.002) and cardiac tamponade (RR = 2.76, 95% CI: 1.15–6.62, p = 0.023). However, this effect was not increased with combined immunotherapy, as was demonstrated in our study.
Fatal cardiac events associated with the use of immune checkpoint inhibitors were recently described in some reports. A meta-analysis by Wang et al. [19] analysed 112 prospective clinical trials that included 19,217 patients with fatal cardiac adverse events associated with 122 deaths: 9 of 58 (16%) with anti-CTLA-4, 4 of 33 (12%) with anti-PD-1, 3 of 12 (25%) with anti-PD-L1, and 4 of 19 (21%) with the combination of anti-PD-1/PD-L1 plus CTLA-4. Then, combined immunotherapy was not associated with an increasing number of deaths due to severe cardiac events.
The meta-analysis of Agostinetto et al. [20] including 14 studies comparing combined immunotherapy with single immunotherapy reported cardiovascular adverse events like myocarditis, acute myocardial infarction, pericarditis, arrhythmias, heart failure, cardiovascular disease, cardiac arrest, and cardiovascular events. The authors described studies published until June 2020 from the PubMed, Medline, and Embase databases and only included 2 of 14 (14.28%) phase-3 RCTs. The authors did not find an increase in the incidence of pooled cardiac events with combined immunotherapy (RR = 1.91, 95% CI: 0.52–7.01, p = 0.329). For myocardial infarction (RR = 0.98, 95% CI: 0.21–4.47, p = 0.978), heart failure (RR = 1.04, 95% CI: 0.25–4.26, p = 0.962), and cardiac arrest (RR = 0.79, 95% CI: 0.16–3.83, p = 0.770), they did not report an increase of the number of these cardiac events with combined immunotherapy, as we demonstrated.
The management of cardiac events is the same as recommended in ACC/AHA guidelines for heart failure, acute coronary syndromes, and arrhythmias [21]. Myocarditis is one of the most dangerous complications of immunotherapy [22]. Combined immunotherapy could be more frequent, as demonstrated in a retrospective study [5] but not in a previous meta-analysis [20]. For myocarditis, an early stabilization and control of the symptoms are needed, considering immunotherapy cessation and the use of corticosteroids or second-line type of agents (infliximab, anti-thymocyte globulin, tacrolimus, intravenous immunoglobulin, plasmapheresis, or mycophenolate mofetil) [5].
Our study has some limitations. First, the number of cardiac events may be underreported in several RCTs due to the absence of systematic monitoring of cardiovascular complications. Only events associated with signs or symptoms were likely to be included. We found cardiac events in the supplementary data of each trial or the information provided on clinicaltrials.gov. Second, we only included phase III RCTs and a subpopulation of cancers we considered the most common, like melanoma and lung cancer. However, our findings may be different in other types of cancer. Third, there may be differences between members of a therapeutic class, but the insufficient number of adverse events in each group does not allow comparisons between them. Fourth, in some cases, comparators in monotherapy were different: anti-PD-1, anti-PD-L1, or CTLA-4 inhibitors, which may have had different cardiac safety profiles. Fifth, the information on the frequency of cardiovascular risk factors or previous cardiovascular disease in the treated populations was not adequately described, limiting the identification of groups with a higher risk of cardiac adverse events. Sixth, our study only determines cardiac adverse events in the short and medium term; long-term events are still unknown. Finally, myocarditis was not reported in the included RCTs.
In conclusion, our study reaffirms that the incidence of cardiac adverse events was not increased with the use of combined immunotherapy compared to single immunotherapy, with information obtained from RCTs published until August 2021, including the most frequent types of cancer where immunotherapy is recommended, as was previously described by other authors, and performing the GRADE assessment of the certainty of the evidence for all outcomes. Moreover, we included a large group of outcomes compared with previous studies, including atrial fibrillation and atrial flutter. However, we need more information on myocarditis cases, which are not commonly reported in these clinical trials.