Introduction
Hepatoblastoma (HB) is a destructive liver malignancy, found mostly in children [1–3]. The incidence of HB has increased rapidly in the past few years with higher incidence rates among children aged 0–4 years. Children aged > 15 years are rarely found with HB [4]. The risk factors associated with the development of HB have not been fully understood. Tobacco consumption, low body weight and a few inherited syndromes including the Beckwith-Wiedemann syndrome may be considered as major risk factors contributing to HB development [5]. Complete surgical resection of the tumor remains the cornerstone in HB management and it is associated with higher efficacy and increased survival chances. However, extensive uni/multi focal primary tumor, disease relapse, distant metastasis and lost surgery chances contribute to a poor 5-year survival rate (20–30%) [6, 7]. The last four decades have seen potential advances in the understanding and treatment of cancer. In one such attempt, doxorubicin (DO) and cisplatin (PLA) were used synergistically to manage HB, which led to enhanced prognosis. Thus, lack of effective treatment strategies and anti-HB chemopreventives have started a hunt for novel anti-HB therapeutic sulforaphanes. Isothiocyanates are a naturally occurring set of glucosinolates prevailing among cruciferous vegetables including cabbage and broccoli. Sulforaphane, an active isothiocyanate, shows diverse biological and medicinal properties [8]. Sulforaphane has been reported to have anticancer properties as well as acting as a prime inducer of phase II enzymes [9–11]. Sulforaphene, an analog of sulforaphane, has also been reported to suppress carcinogenesis via the MAP kinase pathway [12]. Though sulforaphane bears significant anticancer potential with low toxicity, high isolation cost limits its application clinically. Sulforaphane was observed to induce anticancer effects against epithelial Hep-2 carcinoma cells and Barrett’s adenocarcinoma [13, 14]. Sulforaphane has been reported to exhibit anticancer activity due its apoptosis- as well as autophagy-inducing potential [15–17]. In addition, sulforaphane has shown synergistic effects with anticancer activity of taxanes, thereby eliminating the stem cells in triple negative breast carcinoma. Thus, taking into consideration the reported anticancer activity of sulforaphane, the current study was designed to investigate its anticancer behavior against HB. In addition, sulforaphane was testified for apoptosis induction, autophagy induction and inhibition of the β-catenin signaling pathway.
Material and methods
Determination of cellular viability
MTT assay was performed to examine the cellular viability of HepU1, HepU2 and normal THLE-2 cells after sulforaphane treatment. Each cell line was placed on 96-well flat bottom microtiter cultural plates with a concentration of 4 × 104 cells/well and precultured for a period of 24 h with incubation. Afterwards, all three cell lines were subjected to sulforaphane treatment (2.5, 5, 10, 20, 40, 80, 160, 320 and 640 μM) in CO2 (5%) incubator for 24 h and at 37°C. Each well of the culture plate was then supplied with 0.2 mg/ml of fresh MTT solution. After 4 h of additional incubation at 37°C, detergent solution DMSO was used for dissolving formazan crystals. Finally, an Asys UVM 340 Microplate reader, Biochrom, was used to take absorbance at 570 nm for the measurement of optical density (OD).
Clonogenic assay
For clonogenic assessment of HepU1 and HepU2 cell lines after sulforaphane exposure a clonogenic assay was implemented. Briefly, both the cancerous cell lines were loaded onto 6-well plates with 300–400 cells/well. Afterwards, each well was supplemented with varying sulforaphane doses of control, 5, 10 and 20 μM for 10 days. After 10 days of exposure when cell colonies became visible, complete medium was removed and cell colonies were washed three times using PBS (phosphate buffer saline). Both types of cell lines were stained for 15 min with crystal violet after fixation with paraformaldehyde (4%). Clusters of ≥ 50 cells were considered as cell colonies and were photographed with a camera.
Annexin V/PI staining
Quantification of apoptosis in HepU1 and HepU2 cells was accomplished by performing annexin V/PI staining assay. For that the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences) was used in accordance with provided guidelines. In brief, sub-culture of both cell types at 2.5 × 104 concentration was performed in 6-well plates. Afterwards, different sulforaphane doses, viz control, 5, 10 and 20 μM, were added each plate for a period of 24 h. Thereafter, both treated cell types were harvested and subjected to PBS washing twice followed by resuspension in 1 × binding buffer. Aliquots of 104 cells were subjected to FITC Annexin V and PI (5 μl each) staining. Finally, a flow cytometer (BD Biosciences, United States) was used to examine each sample.
Immunofluorescence for cleaved caspase-3 analysis
The sulforaphane (0, 5 10 and 20 μM) treated HepU1 and HepU2 cells were cultured and subjected to fixation on coverslips with paraformaldehyde for 20 min at 25°C. This was followed by washing with PBS incubation for 30 min with 1% bovine serum albumin (BSA) to prevent the blocking of antibodies non-specifically. The cells were then subjected to incubation with primary antibody (1 : 200 dilution) and washing with PBS. Next the cell samples were subjected to incubation with Alexa Fluor 488-conjugated goat antirabbit IgG secondary antibody (1 : 200) in 1% BSA for 1 h in the dark at 25°C. The samples were then subjected to counterstaining with DAPI for 7 min. Finally, the coverslips were subjected to visualization using a fluorescence microscope (200x magnification).
Autophagy detection
The GFP-LC3 vectors were transfected into the HepU1 and HepU2 cells and then treated with different doses of sulforaphane (0, 5, 10 and 20 μM) and then examined by fluorescence microscopy.
Wound healing migration assay
Wound healing migration assay was performed to monitor the rate of migration in HepU1 and HepU2 cells after sulforaphane exposure (control, 5, 10 and 20 μM). Both the cell lines were cultured in 6-well plates until they reached 80% of growth confluence followed by PBS washing once. Thereafter, a sterile pipette tip was used to scratch a wound in both types of treated cell lines including controls and pictured. All well plates were then incubated at 37°C for 24 h followed by taking further images with an inverted microscope.
Transwell chambers invasion assay
Transwell chambers were coated with Matrigel in order to assess cell invasion in HepU1 and HepU2 cells after test sulforaphane treatment. Briefly, both the cell lines were transfected for 24 h with sulforaphane with various concentrations, viz control, 5, 10 and 20 μM. Afterwards, 200 ml of transfected cells were plated onto upper transwell chambers containing medium and 10% FBS (fetal bovine serum). The bottom chambers were only plated with medium. After 2 h of incubation, non-invaded cells were removed, and invaded cells were fixed using methyl alcohol followed by staining with crystal violet (CV). Finally, to estimate the number of invaded cells, an inverted microscope with 200× magnification was used.
Western blotting assay
After exposure with sulforaphane, HepU1 and HepU2 cell lines were lysed using RIPA buffer (ice-cold). Normalization of the lysates was accomplished by the Enhanced BCA Protein Assay Kit (Beyotime). 10–15% SDS-PAGE was used to separate proteins and proteins were electrophoretically transferred to PVDF membranes. Then blocking of membranes was performed using non-fat milk (5%) followed by blotting of membranes with primary antibodies overnight at 4°C (LC3B-I, II, Beclin-1, Atg7, Bax, Bcl-2, caspase-3, PARP, β-catenin and cyclin D1). Species-specific peroxidase-conjugated secondary antibodies were used to detect proteins after incubation. A chemiluminescence Kit (Millipore, Plano, TX, USA) was used to detect immunoreactive bands and visualization was done with a Vilber Fusion FX6-XT imaging system.
Results
Inhibition of HB growth by sulforaphane
Cell growth rate in sulforaphane treated HB cells (HepU1 and HepU2) and treated normal liver cells (THLE2) was monitored through MTT assay. The results demonstrated that the proliferation rate in sulforaphane-treated cancer cell lines (HepU1 and HepU2) was suppressed potentially and dose reliantly. The viability in the case of HepU1 cells decreased from 100% to almost 5% at a sulforaphane concentration varying from 0–640 μM (Figure 1 A). In the case of sulforaphane treated HepU2 cells, viability was observed to decrease remarkably from 100% to almost 2% (Figure 1 B). Against the normal THLE-2 liver cell line, sulforaphane-induced cytotoxicity was comparatively lower (Figure 1 C). Therefore, MTT assay results revealed the antiproliferative nature of sulforaphane against HB. Clonogenic assay indicated that after 10 days of sulforaphane treatment the numbers of cancer cell colonies (both HepU1 and HepU2 cell colonies) were significantly decreased. The number of colonies decreased to almost to 20% in HepU1 (Figure 1D) and almost to 8% on sulforaphane treatment (Figure 1 E).
Figure 1
A – Viability of HepU1 cells after treatment with indicated sulforaphane doses. B – Viability of HEepU2 cells after treatment with indicated sulforaphane doses. C – Viability of THLE-2 cells after treatment with indicated sulforaphane doses. D – Representation of number of HepU1 cell colonies after indicated HEPU1 sulforaphane doses. E – Representation of number of HepU2 cell colonies after indicated HepU1 sulforaphane doses. All the individual experiments were performed three times and the results were stated as mean ± SD (standard deviation) (*p < 0.05)
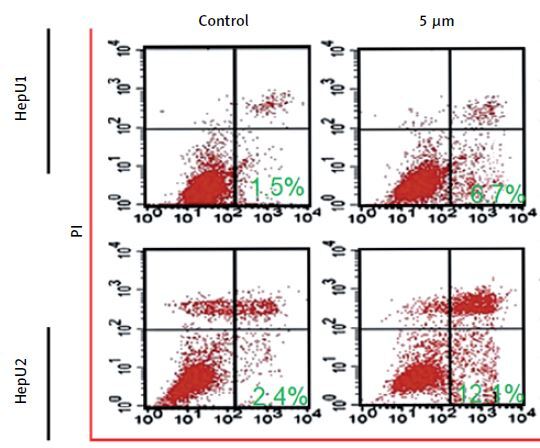
Sulforaphane induced apoptosis in HB cell lines
Sulforaphane induced antiproliferative effects on two different HB cell lines, HepU1 and HepU2; therefore, an attempt was made to reveal its underlying mechanism. Annexin V/PI staining assay was performed to check different quantities of apoptotic HepU1 and HepU2 cells after sulforaphane treatment. In both the cell lines, sulforaphane triggered the induction of apoptotic cell death. The apoptosis percentage was 1.5%, 6.7%, 12.7% and 19.11% in the case of HepU1 and 2.4%, 12.1%, 17.7% and 24.9% at 0, 5, 10 and 20 μM dosage of sulforaphane (Figure 2). Therefore, it was evidenced that anti-cell growth effects of sulforaphane are apoptosis mediated. Further, a DAPI staining assay was performed to monitor nuclear morphology in the case of both the cell lines. The results indicated nuclear fragmentation and enhanced fluorescence indicated higher amounts of cleaved caspase-3 (Figure 3 A) suggestive of apoptotic cell death in HepU1 and HepU2 cell lines after sulforaphane exposure. To further verify the apoptosis-inducing tendency of sulforaphane, western blotting assay was performed. The results revealed that the levels of PARP and caspase-3 remained unchanged in both treated cell lines but cleaved PARP and cleaved caspase-3 showed enhanced expression. It was also observed that the expression of Bcl-2 was suppressed significantly and that of Bax enhanced tremendously on sulforaphane application (Figure 3 B).
Figure 2
Annexin V/PI staining assay results through flow cytometry. Results presenting early, late apoptosis and necrotic HepU1 and HepU2 cell populations at indicated sulforaphane doses. All the individual experiments were performed three times
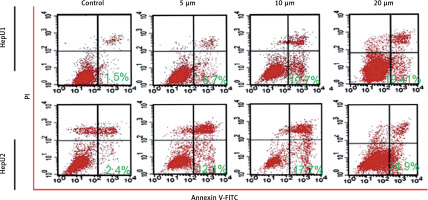
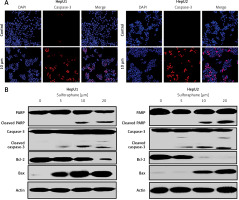
Figure 3
A – Images representing the results of immunofluorescent imaging showing the induction of cleaved caspase-3 in HepU1 and HepU2 cells after sulforaphane treatment. B – Western blotting assay representing activity of pro-apoptotic and anti-apoptotic proteins. All experiments were performed three times
Sulforaphane induced autophagy in HB cells
Sulforaphane was evaluated for its autophagy-inducing potency as well. Enhanced activity of LC3 proteins and autophagosome formation clearly hallmark the autophagic cell death. Fluorescence microscopy indicated increased LC3 proteins which indicated autophagic cell death in both HepU1 and HepU2 cell lines (Figure 4 A). Further, western blotting assay was performed to monitor autophagy-associated protein expression. The results in both cell lines indicated that the activity of Atg-7, Beclin-1, LC3-I and LC3-II was enhanced with increased sulforaphane concentrations. Thus, these results indicate the autophagy-inducing potency of sulforaphane.
Sulforaphane inhibits cell migration and invasion of HB cells
Cell migration was monitored through wound width determination after sulforaphane exposure. The results from the wound healing assay revealed reduced migratory potency of HepU1 and HepU2 cells upon sulforaphane treatment (Figure 5 A). The width of the wound in the case of controls began to almost close after 24 h and a fresh scratched wound was observed in the case of treated cell lines. Cell invasion in both types of cell lines was monitored in transwell chambers coated with Matrigel. The percentage of invaded cells in controls was taken as 100% and the rate of invasion was observed to be declining with increased doses of sulforaphane (Figure 5 B).
Sulforaphane inhibits β-catenin signaling pathway
As the β-catenin signaling pathway is an important pathway in cellular growth and differentiation, targeting it in a cancer cell results in cell death. The β-catenin signaling pathway in HepU1 and HepU2 cells was monitored by western blotting assay. The results indicated that the protein expression of β-catenin, p-β-catenin and cyclin D1 decreased sulforaphane dose dependently (Figure 6). Therefore, the results suggested that the sulforaphane blocked the β-catenin signaling pathway in both HepU1 and HepU2 cell lines.
Discussion
HB is a lethal liver malignancy occurring in children. The disease has been reported with higher incidence in the past decade, globally. Lack of treatment strategies and potential chemo-preventive agents necessitates the development of efficient chemotherapeutic agents. Eukaryotes possess only one known machinery to disintegrate the large intracellular protein aggregates and organelles that cannot be disposed of by the proteasome [18–20]. This machinery is termed “autophagy” and operates through lysosomal degradation. Hence, it is not surprising that microorganisms such as bacteria, protozoa and viruses that infect eukaryotes are degraded by autophagy [21, 22]. Autophagy allied proteins also provide miscellaneous features of immunity in multicellular organisms. Several proteins that regulate autophagy have been identified (around 32) in yeast. In autophagy and phagophore formation, the role of class III PI-3 kinases (notably Vps34 and its binding partner Atg6/Beclin-1) is comparatively well understood [23]. Vps34 plays an important role in membrane sorting in a cell but when Vps34 is complexed with Beclin-1 it exhibits active participation in autophagy. Formation of autophagosomes and upregulation of autophagy allied proteins such as Atgs, Beclin-1 and LC3 proteins hallmark the autophagic cell death [24, 25]. Similarly, apoptosis is another form of cell regulatory mechanism used to discard old, damaged, and malfunctioning cells. Apoptosis is mostly characterized by nuclear condensation, DNA fragmentation, membrane blebbing and plasma membrane rupture [26–28]. It is allied with increased activity of Bax (pro-apoptotic protein) and reduced Bcl-2 (anti-apoptotic) activity. During apoptosis the caspases are activated and initiate the intrinsic pathway of apoptosis. As the apoptosis-inducing potential of sulforaphane was already known, its applications were investigated on HB. Therefore, the current study was instigated to evaluate the anticancer cancer potential of sulforaphane in human HB. Cellular viability of cancerous HepU1 and HepU2 and normal THLE-2 cells was determined by MTT assay, indicating potential viability and colony inhibition in the case of cancerous cells in contrast to normal cells via induction of apoptosis and autophagy. These findings are in agreement with previous studies wherein sulforaphane has been found to inhibit growth of the HEp-2 human epithelial carcinoma cell line [13]. In yet another study, Gamet-Payrastre et al. reported that sulforaphane treatment induces apoptosis in human colon cancer cells, further confirming our results [15]. The β-catenin signaling pathway is an important pathway, and drugs are being developed to target this pathway for the treatment of different cancers. Herein we found that sulforaphane blocks this pathway in HB cells. In addition to cell migration, cell invasion was also impaired by sulforaphane application. These findings are consistent with a previously carried out study wherein sulforaphane was shown to inhibit glioblastoma cell invasion [29].
In conclusion, the data obtained in the present study indicated that sulforaphane exerts anticancer effects on human HB cells. The anticancer effects were found to be mediated via induction of apoptosis, autophagy and inhibition of the β-catenin signaling pathway. Therefore, sulforaphane is a promising molecule in in vitro studies, which makes this substance a good candidate for further research in hepatoblastoma management.