Introduction
Dementia is a prevalent neurodegenerative disorder distinguished by cognitive dysfunction and gradual deterioration in daily functioning [1]. The World Health Organization has recognized dementia as the seventh most common cause of death globally. As the global population ages, the current estimate of over 50 million individuals affected by dementia is projected to increase to 152 million by the year 2050 [2]. Dementia is a multifaceted neurological syndrome characterized by deficits in cognition and memory, with specific subtypes including Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), Parkinson’s disease dementia (PDD), frontotemporal dementia (FTD), and vascular dementia (VD) [3, 4]. The high occurrence of dementia presents considerable obstacles in healthcare, financial resources, and caregiver burden [5]. Thus, the identification of risk factors and biomarkers is essential for the prevention and management of dementia.
The human gut microbiota (GM) consists of 100 trillion microorganisms with over 3 million genes that impact human physiology, health, and behavior [6]. Recent studies have revealed that the GM is important for the nervous system and is connected to neurodegenerative diseases through the microbiota-gut-brain axis (MGBA) [7]. The dysregulation of the MGBA may induce neuroinflammation, lipid metabolic disorder, and synaptic impairment, and subsequently cause cognitive decline [8]. Moreover, the GM offers great potential as a reservoir for new therapeutic opportunities, enabling treatment of numerous neurological disorders by targeting the MGBA [9].
Plasma lipids, commonly assessed through high/low-density lipoprotein cholesterol, triglycerides, and total cholesterol, have been identified as significant factors associated with dementia in numerous studies [10, 11]. Nevertheless, advancements in lipidomics technologies have greatly expanded our comprehension of the diversity and breadth of circulating lipids. Lipid species such as phosphatidylcholine, sterol ester, ceramide and phosphatidylethanolamine have the potential to enhance dementia risk evaluation beyond traditional lipid measures [12, 13].
Lipids play a vital role in cellular function by contributing to membrane structure, intercellular communication, energy storage, and homeostasis regulation [14]. Neurodegenerative diseases and other neurological disorders have been linked to dysregulation of brain lipids [15]. Recent studies have suggested that the GM may influence lipid profiles and lipidomics in dementia, implicating both the GM and lipidome in dementia pathogenesis [16]. It is hypothesized that the lipidome could serve as a mediator in the pathway linking GM to the development of dementia. MR was used in this study to investigate causal relationships between exposure variables and outcomes using single nucleotide polymorphisms (SNPs) as instrumental variables (IVs). The two-sample MR technique enhances statistical power by leveraging published summary estimates from diverse genome-wide association study (GWAS) to identify causal effects between exposure variables and outcomes [17].
This study hypothesized that certain types of lipids may mediate the associations between the gut microbiota and the development of different types of dementia. Therefore, MR analysis was employed to explore the links between the GM, plasma lipidome, and different types of dementia. It also investigated the potential role of the plasma lipidome in the pathway from the GM to dementia, and analyzed the impact of genetic predisposition to dementia risk on the GM and plasma lipidome.
Material and methods
Study design
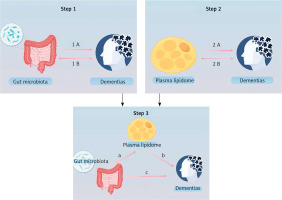
This study comprises three primary components: an examination of the influence of GM on dementia, an investigation into the impact of plasma lipids on dementia, and an analysis of the role of plasma lipids in the pathway from GM to dementia. The study used SNP as IVs and followed the fundamental assumptions of MR [18]. The MR study was reported according to the MR-STROBE guidelines (Figure 1).
Figure 1
Overview of the study (by Figdraw 2.0). Step 1 describes the bi-directional causal effects between GM and dementia. Step 2 describes the bi-directional causal effects between the plasma lipidome and dementia. In Step 3, the mediation analysis of the plasma lipidome from the GM to dementia is outlined
Data source
The data on the GM are derived from Esteban et al.’s study, which reported 412 microbes [19]. The genetic data for the plasma lipidome came from a GWAS with 7174 individuals and 179 lipid species [20].
Data on VD, AD, PDD, FTD, and DLB were collected from FinnGen’s tenth version (https://r10.risteys.finngen.fi/) and Chia’s study [21]. Patients were screened using ICD diagnosis codes for dementia subtypes and genetic data were downloaded from the FinnGen database. DLB data were included in the IEU Open GWAS (https://gwas.mrcieu.ac.uk/) database based on Chia’s study. Participants were diagnosed using consensus criteria.
GWAS summary statistics were used as secondary data in the study, following ethical guidelines. Ethical approval was obtained for the original studies, and the results can be accessed on the website provided. The data are publicly available and do not require further ethical review.
Selection of instrumental variables
The genetic instruments met the following criteria: (1) we selected the SNPs with a p- value of 1 × 10–5 for GM as the threshold; (2) we selected the SNPs with significant associations for the plasma lipidome (p < 5 × 10–8); (3) we demonstrated independent association linkage disequilibrium (LD) clumping r2 < 0.001 and distance > 10,000 kb; (4) we removed palindromic SNPs (SNP with the A/T or G/C alleles) after matching the outcome.
Mendelian randomization analysis
In this study, we used 412 GM taxa and 179 plasma lipid species as exposure factors for dementia, analyzed using MR with the TwoSampleMR package. We used the IVW method to assess causal relationships, presenting results with odds ratios and 95% confidence intervals. Statistical significance was determined with a p-value less than 0.05 for the IVW method, with consistency in direction between IVW and MR-Egger. Suggestive associations were those with a p-value below 0.05 but above the Bonferroni-corrected threshold.
In the mediation analysis, this study identified the GM and plasma lipidome as having significant causal effects on dementias through two-sample analysis. The investigation aimed to determine whether GM had a causal impact on plasma lipidome, and subsequently utilized multiple MR analyses to assess whether the plasma lipidome acted as a mediator in the pathway from the GM to dementia. Bi-directional causal effects were tested between the GM, plasma lipidome and dementias. We employed dementias as the “exposure” variable and identified GM or plasma lipidome linked to dementias as the outcome variables. We utilized SNPs that were found to be significantly correlated with dementia (p < 5 × 10–8) as IVs.
We tested heterogeneity in IVW estimates with Cochran’s Q test and visualized MR results with scatter plots. We conducted sensitivity analyses and used MR-PRESSO and MR-Egger regression to check for horizontal pleiotropy [22]. MR-PRESSO was used to identify and correct outliers, addressing horizontal pleiotropy effects [23]. The Steiger directionality test was used to establish causality, identifying genetic variants with stronger correlations with the outcome than the exposure. Variants identified by the Steiger test were excluded from subsequent analysis. Analysis of MR was carried out using the R package TwoSampleMR (version 4.3.2).
Results
A total of 2774 SNPs were chosen as IVs for the 412 GM taxa and bacterial pathways (Supplementary Table SI). Subsequently, 601 SNPs were identified as being associated with 179 plasma lipid species (Supplementary Table SII).
Causal effects of GM and plasma lipidome on multiple dementia types
AD
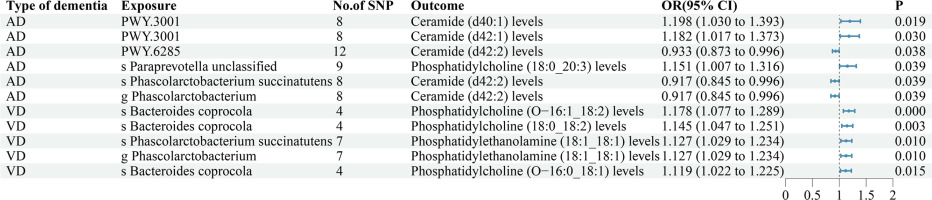
The findings of our study demonstrated that 8 GM taxa and 11 bacterial pathways were associated with AD (Supplementary Table SIII, Figure 2). MR analysis suggested that genetic prediction of 6 GM taxa and 4 bacterial pathways were positively correlated with AD. The species Desulfovibrio piger (OR = 1.206, p = 0.003), species Paraprevotella unclassified (OR = 1.162, p = 0.035), species Alistipes indistinctus (OR = 1.223, p = 0.002), genus Escherichia (OR = 1.185, p = 0.029), family Clostridiales noname (OR = 1.227, p = 0.006), genus Paraprevotella (OR = 1.154, p = 0.016), PWY.66.422(OR = 1.180, p = 0.033), PWY.5188(OR = 1.325, p < 0.001), NAGLIPASYN.PWY(OR = 1.170, p = 0.048) and NONOXIPENT.PWY (OR = 1.230, p = 0.036) may increase the risk of developing AD.
Genetic prediction of 2 GM taxa and 7 bacterial pathways were negatively correlated with AD. The species Phascolarctobacterium succinatutens (OR = 0.897, p = 0.018), genus Phascolarctobacterium (OR = 0.897, p = 0.018), PWY.7392 (OR = 0.864, p = 0.001), PWY.6285 (OR = 0.917, p = 0.029), PRPP.PWY (OR = 0.860, p = 0.036), PWY.3001(OR = 0.728, p = 0.008), PWY.5104 (OR = 0.817, p = 0.014), PWY.5686 (OR = 0.705, p = 0.001) and PENTOSE.P.PWY(OR = 0.868, p = 0.035) may reduce the risk of developing AD.
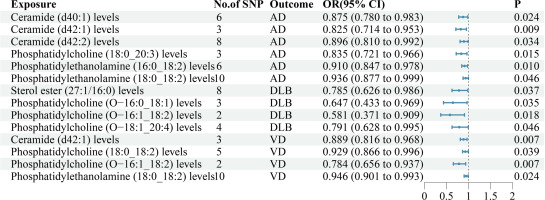
The findings of our study demonstrated that 6 lipid species exhibit associations with AD (Supplementary Table SIV, Figure 3). MR analysis suggested that genetic prediction of all 6 lipid species were negatively correlated with AD. Ceramide (d40:1) levels(OR = 0.875, p = 0.024), ceramide (d42:1) levels(OR = 0.825, p = 0.009), ceramide (d42:2) levels(OR = 0.896, p = 0.034), phosphatidylcholine (18:0_20:3) levels(OR = 0.835, p = 0.015), phosphatidylethanolamine (16:0_18:2) levels(OR = 0.910, p = 0.010) and phosphatidylethanolamine (18:0_18:2) levels(OR = 0.936, p = 0.046) may reduce the risk of developing AD.
FTD
The findings of our study demonstrated that 12 GM taxa and 4 bacterial pathways were associated with FTD (Supplementary Table SIII, Figure 2). MR analysis suggested that genetic prediction of 9 GM taxa and 3 bacterial pathways were positively correlated with FTD. The species Adlercreutzia equolifaciens (OR = 3.024, p = 0.026), species Lactobacillus delbrueckii (OR = 1.450, p = 0.034), genus Escherichia (OR = 3.613, p = 0.010), order Enterobacteriales (OR = 9.328, p = 0.001), class Gammaproteobacteria (OR = 4.844, p = 0.039), family Eubacteriaceae (OR = 4.813, p = 0.030), family Enterobacteriaceae (OR = 9.324, p = 0.001), genus Adlercreutzia (OR = 3.016, p = 0.026), genus Eubacterium (OR = 4.815, p = 0.030), PWY.6151 (OR = 3.234, p = 0.009), HOMOSER.METSYN.PWY (OR = 2.998, p = 0.036) and P23.PWY (OR = 1.640, p = 0.039) may increase the risk of developing FTD.
Genetic prediction of 3 GM taxa and 1 bacterial pathway were negatively correlated with FTD. The species Bilophila unclassified (OR = 0.277, p = 0.001), species Bacteroides thetaiotaomicron (OR = 0.189, p = 0.001), species Lachnospiraceae bacterium 5_1_63FAA (OR = 0.572, p = 0.037), and ANAEROFRUCAT.PWY (OR = 0.174, p = 0.025) may reduce the risk of developing FTD.
No lipid species were associated with FTD.
DLB
The findings of our study demonstrated that 3 GM taxa and 2 bacterial pathways were associated with DLB (Supplementary Table SIII, Figure 2). MR analysis suggested that genetic prediction of 2 GM taxa were positively correlated with DLB. The genus Roseburia (OR = 1.340, p = 0.033) and genus Parabacteroides (OR = 1.382, p = 0.018) may increase the risk of developing DLB.
Genetic prediction of 1 GM taxon and 2 bacterial pathways were negatively correlated with DLB. The species Escherichia coli (OR = 0.775, p = 0.014), PWY.5101 (OR = 0.758, p = 0.049) and PWY.5705 (OR = 0.756, p = 0.001) may reduce the risk of developing DLB.
The findings of our study demonstrated that 4 lipid species exhibit associations with DLB (Supplementary Table SIV, Figure 3). MR analysis suggested that genetic prediction of all 4 lipid species was negatively correlated with DLB. Sterol ester (27:1/16:0) levels (OR = 0.785, p = 0.037), phosphatidylcholine (O−16:0_18:1) levels (OR = 0.647, p = 0.035), phosphatidylcholine (O−16:1_18:2) levels (OR = 0.581, p = 0.018) and phosphatidylcholine (O−18:1_20:4) levels (OR = 0.791, p = 0.046) may reduce the risk of developing DLB.
PDD
The findings of our study demonstrated that 6 GM taxa and 11 bacterial pathways were associated with PDD (Supplementary Table SIII, Figure 2). MR analysis suggested that genetic prediction of 4 GM taxa and 6 bacterial pathways were positively correlated with PDD. The phylum Proteobacteria (OR = 1.643, p = 0.026), species Bifidobacterium bifidum (OR = 1.275, p = 0.025), family Clostridiaceae (OR = 1.461, p = 0.021), genus Lactobacillus (OR = 1.356, p = 0.009), PWY.7196 (OR = 1.897, p = 0.028), PWY.7315 (OR = 1.641, p = 0.016), PWY.6121 (OR = 1.867, p = 0.050), PWY.1269 (OR = 2.182, p = 0.027), PWY.5384 (OR = 1.733, p = 0.024) and PWY.5695 (OR = 1.767, p = 0.029) may increase the risk of developing PDD.
Genetic prediction of 2 GM taxa and 5 bacterial pathways were negatively correlated with PDD. The species Bacteroides coprocola (OR = 0.719, p = 0.049), species Bacteroides intestinalis (OR = 0.492, p = 0.012), PWY.7400 (OR = 0.568, p = 0.030), SULFATE.CYS.PWY (OR = 0.628, p = 0.049), PWY.5791 (OR = 0.757, p = 0.048), PWY.6147 (OR = 0.644, p = 0.034) and GLYCOCAT.PWY (OR = 0.564, p = 0.004) may reduce the risk of developing PDD.
No lipid species were associated with PDD.
VD
The findings of our study demonstrated that 9 GM taxa and 4 bacterial pathways were associated with VD (Supplementary Table SIII, Figure 2). MR analysis suggested that genetic prediction of 3 GM taxa and 3 bacterial pathways were positively correlated with VD. The species Bacteroides fragilis (OR = 1.058, p = 0.033), species Alistipes senegalensis (OR = 1.108, p = 0.027), species Eubacterium hallii (OR = 1.111, p = 0.001), PWY.GLYOXYLATE.BYPASS (OR = 1.078, p = 0.043), PWY0.1479 (OR = 1.117, p = 0.020) and PWY.5188 (OR = 1.124, p = 0.009) may increase the risk of developing VD.
Genetic prediction of 6 GM taxa and 1 bacterial pathway were negatively correlated with VD. The species Bacteroides clarus (OR = 0.939, p = 0.012), species Bacteroides coprocola (OR = 0.939, p = 0.050), species Ruminococcus torques (OR = 0.917, p = 0.041), species Ruminococcus bromii (OR = 0.870, p = 0.009), species Phascolarctobacterium succinatutens (OR = 0.928, p = 0.020), genus Phascolarctobacterium (OR = 0.928, p = 0.020) and PWY.5850 (OR = 0.957, p = 0.045) may reduce the risk of developing VD.
The findings of our study demonstrated that 4 lipid species exhibit associations with VD (Supplementary Table SIV, Figure 3). MR analysis suggested that genetic prediction of all 4 lipid species were negatively correlated with VD. Ceramide (d42:1) levels (OR = 0.889, p = 0.007), phosphatidylcholine (18:0_18:2) levels (OR = 0.929, p = 0.039), phosphatidylcholine (O−16:1_18:2) levels (OR = 0.784, p = 0.007) and phosphatidylethanolamine (18:0_18:2) levels (OR = 0.946, p = 0.024) may reduce the risk of developing VD.
Sensitivity analyses
Our research found no genetic pleiotropy or horizontal pleiotropy influencing the results, and there was no statistically significant heterogeneity in the dataset (Supplementary Table SV). The “leave-one-out” analysis demonstrated the reliability of the MR analysis. Scatter plots illustrated the collective impact of the GM on dementia. Furthermore, the forest plots showed causal associations between the GM and dementia (Supplementary Figures S1–S8).
Bi-directional causal effects of dementias on GM and plasma lipidome
Based on Supplementary Table SVI, there was no reverse effect between GM or plasma lipidome on DLB, PDD and VD. No SNP can be used as an IV after matching FTD with the GM or plasma lipidome. AD had causal effects on phosphatidylethanolamine (16:0_18:2) levels (OR = 1.063, p = 0.021).
Mediation effect of plasma lipidome
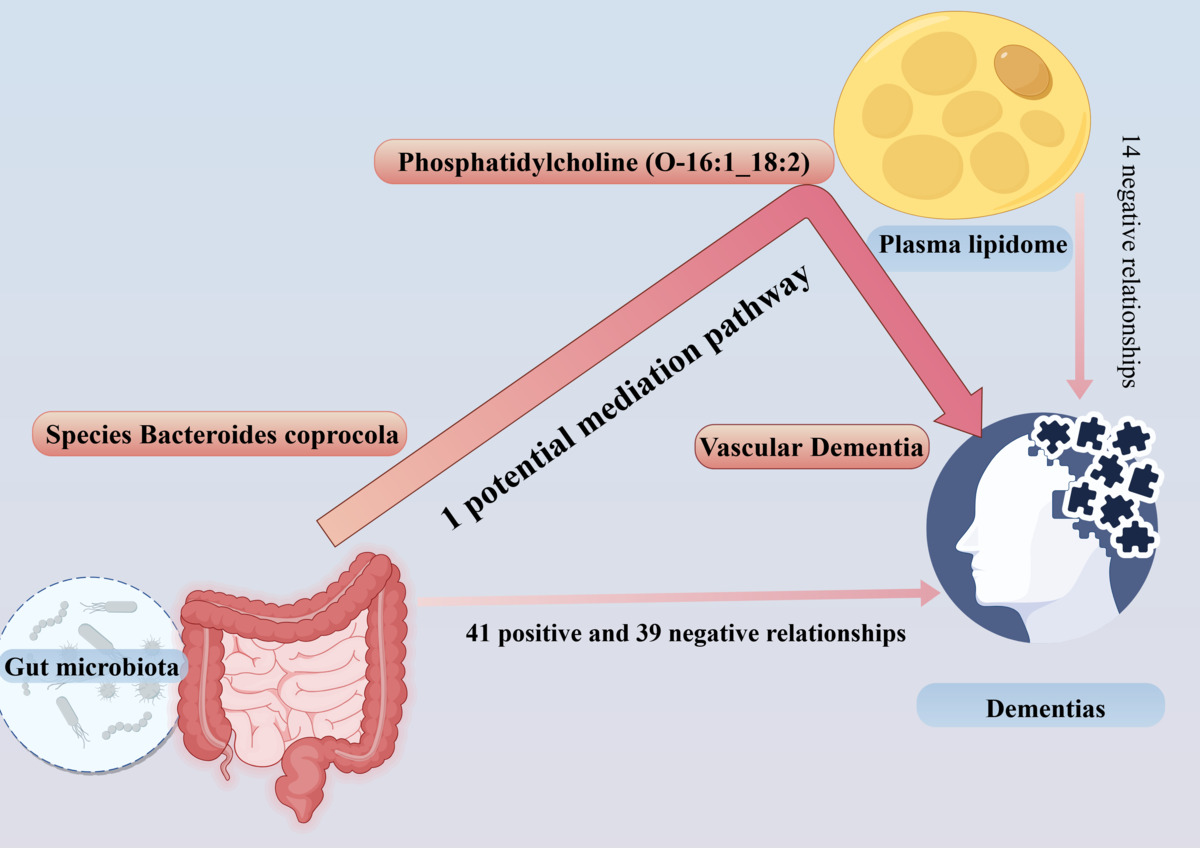
This study discovered that both the gut microbiota (GM) and plasma lipidome play a significant role in the development of dementias. The plasma lipidome may act as a mediator between the GM and dementia, with a key condition being the association between the GM and plasma lipidome. Additional analysis identified 11 potential mediation pathways (Supplementary Table SVII, Figure 4). Ultimately, only 1 potential mediation pathway was found to have a real mediating effect (Table I).
Discussion
In our study, we found that certain GM taxa, bacterial pathways, and plasma lipids are linked to dementia risk. Specifically, phosphatidylcholine (O-16:1_18:2) levels may explain a significant portion of the effect of Bacteroides coprocola on reducing vascular dementia. Our analysis supports a causal relationship between GM taxa, plasma lipids, and dementia.
The GM and gut-derived metabolites play a crucial role in maintaining an individual’s physiological functions, particularly brain functions. The MGBA helps communication between the nervous system and gastrointestinal tract, involving the central nervous system, enteric nervous system, and hypothalamic-pituitary-adrenal axi [24]. Previous research has linked the GM, the plasma lipidome, and dementia, but our study goes further to identify a causal relationship between specific GM taxa, plasma lipid species, and dementia [25].
Our study found a positive correlation between 6 GM taxa and 4 bacterial pathways with AD, and a negative correlation between the genetic prediction of 2 GM taxa and 7 bacterial pathways with AD. Li et al. discovered a decrease in oxidative stress and inflammatory-related GM taxa, like Alistipes and Desulfovibrio, after treating an AD mouse model. They also observed a decrease in Aβ accumulation in the hippocampus and an increase in antioxidation enzyme activity with PC12 cells [26]. Sun et al. conducted a comparative analysis of the GM and metabolome in APPswe/PS1ΔE9(PAP) exhibiting cognitive decline and age-matched controls. Their findings revealed a significant increase in the abundance of Paraprevotella in the GM of the cognitive decline group [27]. For Phascolarctobacterium, there are heterogeneous findings in different studies. In a meta-analysis that included 11 studies, researchers found that the intestinal Phascolarctobacterium of AD patients increased significantly [28]. However, Jemimah’s study found that the intestinal Phascolarctobacterium of AD patients decreased significantly [29]. Galactose degradation (Leloir pathway) has been closely linked to brain senescence and has frequently been used in the construction of AD mouse models [30, 31]. Tynkkynen’s study found a link between isoleucine and reduction of AD risk, supporting our findings on the potential role of MGBA [32]. Our research also showed conflicting effects of the pentose phosphate pathway and its non-oxidative branch on AD, suggesting a possible risk factor in disrupting the complete pathway in the relationship between MGBA and AD.
The results of our study revealed a positive correlation between genetic prediction of 9 GM taxa and 3 bacterial pathways with FTD, as well as a negative correlation between genetic prediction of 3 GM taxa and 1 bacterial pathway with FTD. Yang’s research revealed that Bacteroides thetaiotaomicron significantly contributed to cognitive impairment in a mouse model of dementia [33]. Furthermore, as an enzyme that converts cholesterol to cholesterol-3-sulfonate, Bacteroides thetaiotaomicron played a role in regulating blood cholesterol levels by sulfonating steroidal metabolites, suggesting a potential avenue for mitigating frontotemporal dementia [34]. The findings regarding the impact of Lachnospiraceae on dementia research have been inconsistent. Li’s study suggested that Lachnospiraceae may contribute to excitotoxic effects, metabolic damage, inflammatory responses, and neural and astrocytic apoptosis through quinolinic acid synthesis [35]. Additionally, the study highlights a potential link between the biosynthesis of the methionine-related pathway and the development of FTD. Stopa’s research revealed a notable decrease in the decomposition of methionine in individuals diagnosed with frontotemporal dementia [36].
The results of our study revealed a positive correlation between genetic prediction of 2 GM taxa with DLB, as well as a negative correlation between genetic prediction of 1 GM taxon and 2 bacterial pathways with DLB. Klein found that the functional amyloid fibers produced by Escherichia had a similar structure to alpha synuclein, which was closely related to the pathogenesis of DLB [37]. This study is the first to report that Roseburia and Parabacteroides are risk factors for DLB, which may have potential biological application value.
The findings of our study revealed a positive correlation of the genetic prediction of 4 GM taxa and 6 bacterial pathways with PDD, alongside a negative correlation of the genetic prediction of 2 GM taxa and 5 bacterial pathways with PDD. Chang’s research suggested that Bacteroides may have a positive impact on cognitive function in individuals with Parkinson’s disease by metabolizing D-glutamate [38]. Additionally, Heravi’s study indicated an increase in the expression of Bifidobacterium in patients with PDD, while no significant difference was observed in Proteobacteria levels between PDD patients and the general population [39]. Despite previous beliefs associating Lactobacillus with beneficial effects on health, this study revealed that Lactobacillus may actually be a risk factor for PDD [40]. This finding underscored the importance of further examining the use of certain probiotics in the context of neurological diseases. In terms of protective factors for PDD, our findings were similar to those of recent studies in PD patients [41].
The results of our study revealed a positive correlation between genetic prediction of 3 GM taxa and 3 bacterial pathways with VD, as well as a negative correlation between genetic prediction of 6 GM taxa and 1 bacterial pathway with VD. There is an ongoing debate surrounding the role of Bacteroides in cognitive function. While certain studies had reported a notable increase in Bacteroides within the GM of individuals with vascular dementia and post-stroke cognitive impairment, other research suggested a decrease in Bacteroides among those with cognitive impairment [42, 43].
Our research involved a detailed classification and analysis of Bacteroides, revealing Bacteroides clarus and Bacteroides coprocola as protective factors against VD, while Bacteroides fragilis emerged as a risk factor for VD. Additionally, Wu’s study identified an enrichment of Bacteroides clarus and Bacteroides coprocola in individuals with low levels of indole-3-acetic acid, whereas Bacteroides fragilis was found to be enriched in those with high levels of indole-3-acetic acid, indicating a significant risk factor for vascular cognitive impairment [44, 45]. Xia’s investigation revealed the involvement of Bacteroides fragilis in the activation of microglia and the induction of Alzheimer’s disease pathologies in Thy1-C/EBPβ transgenic mice [46]. In a separate study, Zhao demonstrated that Bacteroides fragilis may contribute to the development of neuroinflammation via lipopolysaccharides, resulting in cognitive impairment [47]. These findings underscore the significance of identifying precise bacterial strains in future research on the MGBA.
This study aimed to investigate the impact of GM taxa on dementia by analyzing their relative abundance expression. However, the precise mechanism underlying the relationship between GM and dementia remains unclear. It was hypothesized that plasma lipid species may serve as mediators in the interaction between the GM and the development of dementia.
MR analysis suggested that genetic predictions of all 6 lipid species were negatively correlated with AD, no lipid species were associated with FTD, all 4 lipid species were negatively correlated with DLB, no lipid species were associated with PDD, and all 4 lipid species were negatively correlated with VD. Interestingly, our research revealed a significant correlation between lipid levels and the reduction of dementia incidence. Numerous studies have demonstrated that maintaining normal lipid metabolism in the central nervous system could greatly decrease the risk of dementia. Ceramide, phosphatidylcholine, phosphatidylethanolamine, and sterol ester all exhibited protective properties against various forms of dementia, a finding supported by multiple studies. Additionally, research suggested that proper metabolism of sphingomyelin and ceramide may facilitate synaptic plasticity and cognitive enhancement [48]. Ylilauri’s research revealed a significant association between increased phosphatidylcholine intake and reduced risk of dementia and enhanced cognitive function [43].
Our research found that phosphatidylcholine (O-16:1_18:2) level mediated the causal effects of the species Bacteroides coprocola on reduction of VD (proportion mediated = 63.6%). While previous research has linked the GM to dementia, the exact ways it affects vascular dementia are still unclear. More research in this area could improve our understanding. Our findings suggest that targeting Bacteroides coprocola could help treat vascular dementia. This approach involves using various treatments such as antibiotics, modified bacteria, prebiotics, and metabolites to control its levels. Further research is needed to understand the role of Bacteroides coprocola in clinical practice.
In conclusion, this study identified important associations between GM taxa, bacterial pathways, plasma lipids, and different types of dementia. These findings offer insights into potential biomarkers and treatment options for these complex diseases. Additionally, the study revealed the diverse mechanisms involved in dementia development, showing that GM taxa can be both protective and risk factors for dementia, emphasizing the complex relationship between microbial communities and disease progression. More research is needed to understand how the GM is connected to dementia. However, there are certain limitations to this study. Firstly, the study population was limited to European individuals, excluding other ethnic groups. Secondly, the specific mechanisms through which the gut microbiota affects the occurrence and progression of dementia via lipids were not elucidated in this study. Our study suggests that altering the GM could help reduce dementia risk and improve patient outcomes. However, more research is necessary to apply these findings in clinical practice.